N, N-Dimethyltryptamine, a natural hallucinogen, ameliorates Alzheimer's disease by restoring neuronal Sigma-1 receptor-mediated endoplasmic reticulum-mitochondria crosstalk
- PMID: 38693554
- PMCID: PMC11061967
- DOI: 10.1186/s13195-024-01462-3
N, N-Dimethyltryptamine, a natural hallucinogen, ameliorates Alzheimer's disease by restoring neuronal Sigma-1 receptor-mediated endoplasmic reticulum-mitochondria crosstalk
Abstract
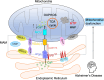
Background: Aberrant neuronal Sigma-1 receptor (Sig-1r)-mediated endoplasmic reticulum (ER)- mitochondria signaling plays a key role in the neuronal cytopathology of Alzheimer's disease (AD). The natural psychedelic N, N-dimethyltryptamine (DMT) is a Sig-1r agonist that may have the anti-AD potential through protecting neuronal ER-mitochondrial interplay.
Methods: 3×TG-AD transgenic mice were administered with chronic DMT (2 mg/kg) for 3 weeks and then performed water maze test. The Aβ accumulation in the mice brain were determined. The Sig-1r level upon DMT treatment was tested. The effect of DMT on the ER-mitochondrial contacts site and multiple mitochondria-associated membrane (MAM)-associated proteins were examined. The effect of DMT on calcium transport between ER and mitochondria and the mitochondrial function were also evaluated.
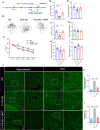
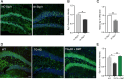
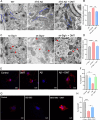
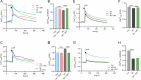
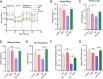
Results: chronic DMT (2 mg/kg) markedly alleviated cognitive impairment of 3×TG-AD mice. In parallel, it largely diminished Aβ accumulation in the hippocampus and prefrontal cortex. DMT restored the decreased Sig-1r levels of 3×TG-AD transgenic mice. The hallucinogen reinstated the expression of multiple MAM-associated proteins in the brain of 3×TG-AD mice. DMT also prevented physical contact and calcium dynamic between the two organelles in in vitro and in vivo pathological circumstances. DMT modulated oxidative phosphorylation (OXPHOS) and ATP synthase in the in vitro model of AD.
Conclusion: The anti-AD effects of DMT are associated with its protection of neuronal ER-mitochondria crosstalk via the activation of Sig-1r. DMT has the potential to serve as a novel preventive and therapeutic agent against AD.
Keywords: Alzheimer’s disease; ER-mitochondria crosstalk; N,N-Dimethyltryptamine; Sigma-1 receptor, calcium homeostasis; cognitive impairment.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Markovinovic A, et al. Endoplasmic reticulum–mitochondria signaling in neurons and neurodegenerative diseases. J Cell Sci. 2022;135(3):pjcs248534. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

