Disorders of fatty acid homeostasis
- PMID: 38693715
- PMCID: PMC11730842
- DOI: 10.1002/jimd.12734
Disorders of fatty acid homeostasis
Abstract
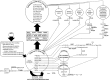
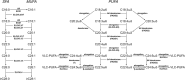
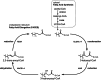
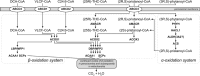
Humans derive fatty acids (FA) from exogenous dietary sources and/or endogenous synthesis from acetyl-CoA, although some FA are solely derived from exogenous sources ("essential FA"). Once inside cells, FA may undergo a wide variety of different modifications, which include their activation to their corresponding CoA ester, the introduction of double bonds, the 2- and ω-hydroxylation and chain elongation, thereby generating a cellular FA pool which can be used for the synthesis of more complex lipids. The biological properties of complex lipids are very much determined by their molecular composition in terms of the FA incorporated into these lipid species. This immediately explains the existence of a range of genetic diseases in man, often with severe clinical consequences caused by variants in one of the many genes coding for enzymes responsible for these FA modifications. It is the purpose of this review to describe the current state of knowledge about FA homeostasis and the genetic diseases involved. This includes the disorders of FA activation, desaturation, 2- and ω-hydroxylation, and chain elongation, but also the disorders of FA breakdown, including disorders of peroxisomal and mitochondrial α- and β-oxidation.
Keywords: (phospho)lipid metabolism; 2‐hydroxylation; fatty acid elongation; mitochondrial disorders; peroxisomal disorders; sphingolipid metabolism; ω‐hydroxylation.
© 2024 The Authors. Journal of Inherited Metabolic Disease published by John Wiley & Sons Ltd on behalf of SSIEM.
Figures

Similar articles
-
Omega-3 fatty acids for depression in adults.Cochrane Database Syst Rev. 2021 Nov 24;11(11):CD004692. doi: 10.1002/14651858.CD004692.pub5. Cochrane Database Syst Rev. 2021. PMID: 34817851 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Exploring the impact of fatty acid composition on carcass and meat quality in Bos taurus indicus influenced cattle.J Anim Sci. 2024 Jan 3;102:skae306. doi: 10.1093/jas/skae306. J Anim Sci. 2024. PMID: 39383295
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Uncommon Non-MS Demyelinating Disorders of the Central Nervous System.Curr Neurol Neurosci Rep. 2025 Jul 1;25(1):45. doi: 10.1007/s11910-025-01432-8. Curr Neurol Neurosci Rep. 2025. PMID: 40591029 Review.
Cited by
-
The Role of Linoleic Acid in Skin and Hair Health: A Review.Int J Mol Sci. 2024 Dec 30;26(1):246. doi: 10.3390/ijms26010246. Int J Mol Sci. 2024. PMID: 39796110 Free PMC article. Review.
-
CPT2 Deficiency Modeled in Zebrafish: Abnormal Neural Development, Electrical Activity, Behavior, and Schizophrenia-Related Gene Expression.Biomolecules. 2024 Jul 26;14(8):914. doi: 10.3390/biom14080914. Biomolecules. 2024. PMID: 39199302 Free PMC article.
-
The neurological pathology of peroxisomal ACBD5 deficiency - lessons from patients and mouse models.Front Mol Neurosci. 2025 Jul 2;18:1602343. doi: 10.3389/fnmol.2025.1602343. eCollection 2025. Front Mol Neurosci. 2025. PMID: 40672445 Free PMC article. Review.
-
Spatial profiling of carbonyl metabolites in diabetic cardiomyopathy by derivatization-assisted ambient mass spectrometry imaging.Anal Bioanal Chem. 2025 Aug;417(19):4481-4491. doi: 10.1007/s00216-025-05969-y. Epub 2025 Jun 23. Anal Bioanal Chem. 2025. PMID: 40551014
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

