Fibrosis and steatotic liver disease in US adolescents according to the new nomenclature
- PMID: 38693784
- PMCID: PMC11938172
- DOI: 10.1002/jpn3.12230
Fibrosis and steatotic liver disease in US adolescents according to the new nomenclature
Abstract
Objective: To apply the new nomenclature for steatotic liver diseases (SLD), replacing nonalcoholic fatty liver disease (NAFLD) with metabolic dysfunction-associated steatotic liver disease (MASLD), in adolescents using National Health and Nutrition Examination Survey (NHANES) data.
Methods: Among 1410 adolescents (12-19 years) in NHANES (2017-March, 2020), the controlled attenuation parameter (CAP) of transient elastography (TE) was used to define steatosis and fibrosis (TE ≥ 7.4 kPa). Obesity and alanine aminotransferase (ALT) ≥ 80 U/L were used to identify adolescents qualifying for hepatology referral according to practice guidelines. NAFLD was defined as liver steatosis without a specific exposure; it has no cardiometabolic risk factor requirement, unlike MASLD.
Results: Steatosis (yes/no) is the first decision point in the new diagnostic protocol; however, criteria for steatosis are undefined. At the supplier (EchoSens)-recommended CAP threshold of 240 dB/m, 30.5% (95% confidence interval [CI]: 27.1%-34.0%) of adolescents had SLD and about 85% of adolescents with NAFLD met criteria for MASLD. The other 15% would receive an ambiguous diagnosis of either cryptogenic SLD or possible MASLD. At higher CAP thresholds, MASLD/NAFLD concordance increased and approached 100%. Among adolescents with MASLD-fibrosis, only 8.8% (95% CI: 0%-19.3%) had overweight/obese and ALT ≥ 80 U/L.
Conclusions: The new nomenclature highlights the high prevalence of liver steatosis. At the CAP threshold of 240 dB/m, however, approximately 15% of adolescents would receive an ambiguous diagnosis, which could lead to confusion and worry. Fewer than 10% of adolescents with MASLD-fibrosis had overweight/obese and ALT ≥ 80 U/L. Revised guidelines are needed to ensure that the other 90% receive appropriate referral and liver disease care.
Keywords: MASLD; NHANES; VCTE; cardiometabolic risk factors; cryptogenic SLD.
© 2024 European Society for Pediatric Gastroenterology, Hepatology, and Nutrition and North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition.
Conflict of interest statement
CONFLICT OF INTEREST STATEMENT
Mount Sinai receives support for Dr. Andrea D. Branch’s research from Gilead and Pfizer. Dr. Andrea D. Branch advised the Center for Disease Analysis Foundation. Dr. Jaime Chu has done ad hoc consulting for Albireo Pharma in the last 1 year. Dr. Meena Bansal has done consulting/Ad boards in Kinetix, Madrigal, Pfizer, Theratechnologies, Fibronostics, and NOVO Nordisk; and Mount Sinai receives support for Dr. Bansal’s research. Dr. Ning Ma had nothing to report.
Figures

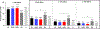
References
-
- American Association for the Study of Liver Diseases, Latin American Association for the Study of the Liver, European Association for the Study of the Liver. A call for unity: the path towards a more precise and patient-centric nomenclature for NAFLD. Hepatology. 2023;78(1):3–5. doi: 10.1097/hep.0000000000000412 - DOI - PubMed
-
- Vos MB, Abrams SH, Barlow SE, et al. NASPGHAN clinical practice guideline for the diagnosis and treatment of nonalcoholic fatty liver disease in children: recommendations from the expert committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J Pediatr Gastroenterol Nutr. 2017;64(2):319–334. doi: 10.1097/mpg.0000000000001482 - DOI - PMC - PubMed
-
- Johnson CL, Paulose-Ram R, Ogden CL, et al. National health and nutrition examination survey: analytic guidelines, 1999–2010. Vital Health Statis Ser 2 Data Eval Methods Res. 2013;161:1–24. - PubMed
-
- National Health and Nutrition Examination Survey 2017-March 2020 Data Documentation. https://wwwncdcgov/Nchs/Nhanes/2017-2018/P_LUXhtm
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

