New synergistic combination therapy approaches with HDAC inhibitor quisinostat, cisplatin or PARP inhibitor talazoparib for urothelial carcinoma
- PMID: 38693852
- PMCID: PMC11063726
- DOI: 10.1111/jcmm.18342
New synergistic combination therapy approaches with HDAC inhibitor quisinostat, cisplatin or PARP inhibitor talazoparib for urothelial carcinoma
Abstract
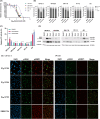
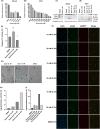
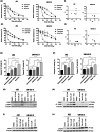
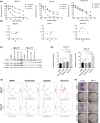
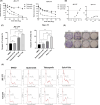
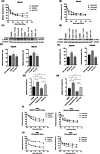
Urothelial carcinoma (UC) urgently requires new therapeutic options. Histone deacetylases (HDAC) are frequently dysregulated in UC and constitute interesting targets for the development of alternative therapy options. Thus, we investigated the effect of the second generation HDAC inhibitor (HDACi) quisinostat in five UC cell lines (UCC) and two normal control cell lines in comparison to romidepsin, a well characterized HDACi which was previously shown to induce cell death and cell cycle arrest. In UCC, quisinostat led to cell cycle alterations, cell death induction and DNA damage, but was well tolerated by normal cells. Combinations of quisinostat with cisplatin or the PARP inhibitor talazoparib led to decrease in cell viability and significant synergistic effect in five UCCs and platinum-resistant sublines allowing dose reduction. Further analyses in UM-UC-3 and J82 at low dose ratio revealed that the mechanisms included cell cycle disturbance, apoptosis induction and DNA damage. These combinations appeared to be well tolerated in normal cells. In conclusion, our results suggest new promising combination regimes for treatment of UC, also in the cisplatin-resistant setting.
Keywords: HDACi; bladder cancer; cisplatin; quisinostat; talazoparib.
© 2024 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Co-targeting poly(ADP-ribose) polymerase (PARP) and histone deacetylase (HDAC) in triple-negative breast cancer: Higher synergism in BRCA mutated cells.Biomed Pharmacother. 2018 Mar;99:543-551. doi: 10.1016/j.biopha.2018.01.045. Epub 2018 Feb 20. Biomed Pharmacother. 2018. PMID: 29902865
-
Histone deacetylase inhibitor, suberoylanilide hydroxamic acid (SAHA), enhances anti-tumor effects of the poly (ADP-ribose) polymerase (PARP) inhibitor olaparib in triple-negative breast cancer cells.Breast Cancer Res. 2015 Mar 7;17:33. doi: 10.1186/s13058-015-0534-y. Breast Cancer Res. 2015. PMID: 25888415 Free PMC article.
-
Synergistic Cytotoxicity of Histone Deacetylase and Poly-ADP Ribose Polymerase Inhibitors and Decitabine in Breast and Ovarian Cancer Cells: Implications for Novel Therapeutic Combinations.Int J Mol Sci. 2024 Aug 26;25(17):9241. doi: 10.3390/ijms25179241. Int J Mol Sci. 2024. PMID: 39273190 Free PMC article.
-
Changes in histone deacetylase (HDAC) expression patterns and activity of HDAC inhibitors in urothelial cancers.Urol Oncol. 2013 Nov;31(8):1770-9. doi: 10.1016/j.urolonc.2012.06.015. Epub 2012 Sep 1. Urol Oncol. 2013. PMID: 22944197
-
Trichostatin A, a histone deacetylase inhibitor, induces synergistic cytotoxicity with chemotherapy via suppression of Raf/MEK/ERK pathway in urothelial carcinoma.J Mol Med (Berl). 2018 Dec;96(12):1307-1318. doi: 10.1007/s00109-018-1697-7. Epub 2018 Oct 4. J Mol Med (Berl). 2018. PMID: 30288546
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

