Improved therapeutic effects on vascular intimal hyperplasia by mesenchymal stem cells expressing MIR155HG that function as a ceRNA for microRNA-205
- PMID: 38693854
- PMCID: PMC11063722
- DOI: 10.1111/jcmm.18351
Improved therapeutic effects on vascular intimal hyperplasia by mesenchymal stem cells expressing MIR155HG that function as a ceRNA for microRNA-205
Abstract
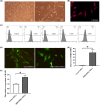
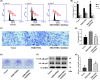
Coronary artery bypass grafting (CABG) is an effective treatment for coronary heart disease, with vascular transplantation as the key procedure. Intimal hyperplasia (IH) gradually leads to vascular stenosis, seriously affecting the curative effect of CABG. Mesenchymal stem cells (MSCs) were used to alleviate IH, but the effect was not satisfactory. This work aimed to investigate whether lncRNA MIR155HG could improve the efficacy of MSCs in the treatment of IH and to elucidate the role of the competing endogenous RNA (ceRNA). The effect of MIR155HG on MSCs function was investigated, while the proteins involved were assessed. IH was detected by HE and Van Gieson staining. miRNAs as the target of lncRNA were selected by bioinformatics analysis. qRT-PCR and dual-luciferase reporter assay were performed to verify the binding sites of lncRNA-miRNA. The apoptosis, Elisa and tube formation assay revealed the effect of ceRNA on the endothelial protection of MIR155HG-MSCs. We observed that MIR155HG improved the effect of MSCs on IH by promoting viability and migration. MIR155HG worked as a sponge for miR-205. MIR155HG/miR-205 significantly improved the function of MSCs, avoiding apoptosis and inducing angiogenesis. The improved therapeutic effects of MSCs on IH might be due to the ceRNA role of MIR155HG/miR-205.
Keywords: MIR155HG; ceRNA; intimal hyperplasia; mesenchymal stem cells; microRNA‐205.
© 2024 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors confirm that there are no conflicts of interest.
Figures





Similar articles
-
The effect of lncRNA MIR155HG-modified MSCs and exosome delivery to synergistically attenuate vein graft intimal hyperplasia.Stem Cell Res Ther. 2022 Nov 4;13(1):512. doi: 10.1186/s13287-022-03197-0. Stem Cell Res Ther. 2022. PMID: 36333764 Free PMC article.
-
The miR155HG/miR-185/ANXA2 loop contributes to glioblastoma growth and progression.J Exp Clin Cancer Res. 2019 Mar 21;38(1):133. doi: 10.1186/s13046-019-1132-0. J Exp Clin Cancer Res. 2019. PMID: 30898167 Free PMC article.
-
Improved therapeutic effects on diabetic foot by human mesenchymal stem cells expressing MALAT1 as a sponge for microRNA-205-5p.Aging (Albany NY). 2019 Dec 21;11(24):12236-12245. doi: 10.18632/aging.102562. Epub 2019 Dec 21. Aging (Albany NY). 2019. PMID: 31866580 Free PMC article.
-
Long noncoding RNA H19 upregulates vascular endothelial growth factor A to enhance mesenchymal stem cells survival and angiogenic capacity by inhibiting miR-199a-5p.Stem Cell Res Ther. 2018 Apr 19;9(1):109. doi: 10.1186/s13287-018-0861-x. Stem Cell Res Ther. 2018. PMID: 29673400 Free PMC article.
-
lncRNA/MicroRNA interactions in the vasculature.Clin Pharmacol Ther. 2016 May;99(5):494-501. doi: 10.1002/cpt.355. Epub 2016 Mar 31. Clin Pharmacol Ther. 2016. PMID: 26910520 Free PMC article. Review.
Cited by
-
Therapeutic Potential and Mechanisms of Mesenchymal Stem Cells in Coronary Artery Disease: Narrative Review.Int J Mol Sci. 2025 Jun 5;26(11):5414. doi: 10.3390/ijms26115414. Int J Mol Sci. 2025. PMID: 40508220 Free PMC article. Review.
References
-
- Dimeling G, Bakaeen L, Khatri J, Bakaeen FG. CABG: when, why, and how? Cleve Clin J Med. 2021;88(5):295‐303. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

