Biocompatible adipose extracellular matrix and reduced graphene oxide nanocomposite for tissue engineering applications
- PMID: 38693996
- PMCID: PMC11061343
- DOI: 10.1016/j.mtbio.2024.101059
Biocompatible adipose extracellular matrix and reduced graphene oxide nanocomposite for tissue engineering applications
Abstract
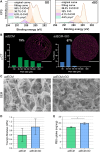
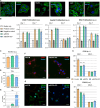
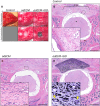
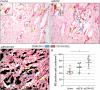
Despite the immense need for effective treatment of spinal cord injury (SCI), no successful repair strategy has yet been clinically implemented. Multifunctional biomaterials, based on porcine adipose tissue-derived extracellular matrix (adECM) and reduced graphene oxide (rGO), were recently shown to stimulate in vitro neural stem cell growth and differentiation. Nevertheless, their functional performance in clinically more relevant in vivo conditions remains largely unknown. Before clinical application of these adECM-rGO nanocomposites can be considered, a rigorous assessment of the cytotoxicity and biocompatibility of these biomaterials is required. For instance, xenogeneic adECM scaffolds could still harbour potential immunogenicity following decellularization. In addition, the toxicity of rGO has been studied before, yet often in experimental settings that do not bear relevance to regenerative medicine. Therefore, the present study aimed to assess both the in vitro as well as in vivo safety of adECM and adECM-rGO scaffolds. First, pulmonary, renal and hepato-cytotoxicity as well as macrophage polarization studies showed that scaffolds were benign invitro. Then, a laminectomy was performed at the 10th thoracic vertebra, and scaffolds were implanted directly contacting the spinal cord. For a total duration of 6 weeks, animal welfare was not negatively affected. Histological analysis demonstrated the degradation of adECM scaffolds and subsequent tissue remodeling. Graphene-based scaffolds showed a very limited fibrous encapsulation, while rGO sheets were engulfed by foreign body giant cells. Furthermore, all scaffolds were infiltrated by macrophages, which were largely polarized towards a pro-regenerative phenotype. Lastly, organ-specific histopathology and biochemical analysis of blood did not reveal any adverse effects. In summary, both adECM and adECM-rGO implants were biocompatible upon laminectomy while establishing a pro-regenerative microenvironment, which justifies further research on their therapeutic potential for treatment of SCI.
Keywords: Cytotoxicity; Extracellular matrix; Foreign body response; Graphene; Macrophages; Nanocomposite.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
Application of Adipose Extracellular Matrix and Reduced Graphene Oxide Nanocomposites for Spinal Cord Injury Repair.Adv Healthc Mater. 2025 Jan;14(3):e2402775. doi: 10.1002/adhm.202402775. Epub 2024 Dec 12. Adv Healthc Mater. 2025. PMID: 39668418 Free PMC article.
-
Interfacing reduced graphene oxide with an adipose-derived extracellular matrix as a regulating milieu for neural tissue engineering.Biomater Adv. 2023 May;148:213351. doi: 10.1016/j.bioadv.2023.213351. Epub 2023 Feb 18. Biomater Adv. 2023. PMID: 36842343
-
Reduced Graphene Oxide-Extracellular Matrix Scaffolds as a Multifunctional and Highly Biocompatible Nanocomposite for Wound Healing: Insights into Characterization and Electroconductive Potential.Nanomaterials (Basel). 2022 Aug 19;12(16):2857. doi: 10.3390/nano12162857. Nanomaterials (Basel). 2022. PMID: 36014722 Free PMC article.
-
Graphene oxide and reduced graphene oxide-based scaffolds in regenerative medicine.Int J Pharm. 2020 Apr 30;580:119226. doi: 10.1016/j.ijpharm.2020.119226. Epub 2020 Mar 13. Int J Pharm. 2020. PMID: 32179151 Review.
-
The influence of reduced graphene oxide on stem cells: a perspective in peripheral nerve regeneration.Regen Biomater. 2021 Jun 25;8(4):rbab032. doi: 10.1093/rb/rbab032. eCollection 2021 Aug. Regen Biomater. 2021. PMID: 34188955 Free PMC article. Review.
Cited by
-
Graphene-Oxide Peptide-Containing Materials for Biomedical Applications.Int J Mol Sci. 2024 Sep 22;25(18):10174. doi: 10.3390/ijms251810174. Int J Mol Sci. 2024. PMID: 39337659 Free PMC article. Review.
-
Application of Adipose Extracellular Matrix and Reduced Graphene Oxide Nanocomposites for Spinal Cord Injury Repair.Adv Healthc Mater. 2025 Jan;14(3):e2402775. doi: 10.1002/adhm.202402775. Epub 2024 Dec 12. Adv Healthc Mater. 2025. PMID: 39668418 Free PMC article.
-
Transplantation of miR-216a-5p-overexpressing mesenchymal stem cells encapsulated in a thermosensitive hydrogel promotes functional recovery in a rat model of spinal cord injury.Eur J Med Res. 2025 Jul 24;30(1):667. doi: 10.1186/s40001-025-02860-5. Eur J Med Res. 2025. PMID: 40708043 Free PMC article.
-
Oxidative stress activates YAP/TEAD1/NCOA4 axis to promote ferroptosis of endplate chondrocytes and aggravate intervertebral disc degeneration.J Orthop Translat. 2025 Jul 12;54:8-25. doi: 10.1016/j.jot.2025.07.001. eCollection 2025 Sep. J Orthop Translat. 2025. PMID: 40688343 Free PMC article.
-
The emerging role of graphene in spinal cord regeneration.Nanomedicine (Lond). 2025 May;20(10):1081-1084. doi: 10.1080/17435889.2025.2475732. Epub 2025 Mar 7. Nanomedicine (Lond). 2025. PMID: 40052204 No abstract available.
References
-
- Gao X., You Z., Li Y., Kang X., Yang W., Wang H., Zhang T., Zhao X., Sun Y., Shen H., Dai J. Multifunctional hydrogel modulates the immune microenvironment to improve allogeneic spinal cord tissue survival for complete spinal cord injury repair. Acta Biomater. 2023;155:235–246. doi: 10.1016/j.actbio.2022.11.015. - DOI - PubMed
-
- Zhu R., Zhu X., Zhu Y., Wang Z., He X., Wu Z., Xue L., Fan W., Huang R., Xu Z., Qi X., Xu W., Yu Y., Ren Y., Li C., Cheng Q., Ling L., Wang S., Cheng L. Immunomodulatory layered Double hydroxide nanoparticles enable neurogenesis by targeting transforming growth factor-β receptor 2. ACS Nano. 2021;15(2):2812–2830. doi: 10.1021/acsnano.0c08727. - DOI - PubMed
LinkOut - more resources
Full Text Sources

