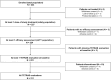
Impact of Switching From Immediate- or Prolonged-Release to Once-Daily Extended-Release Tacrolimus (LCPT) on Tremor in Stable Kidney Transplant Recipients: The Observational ELIT Study
- PMID: 38694490
- PMCID: PMC11061389
- DOI: 10.3389/ti.2024.11571
Impact of Switching From Immediate- or Prolonged-Release to Once-Daily Extended-Release Tacrolimus (LCPT) on Tremor in Stable Kidney Transplant Recipients: The Observational ELIT Study
Abstract
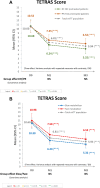
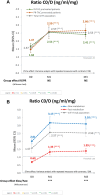
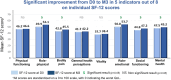
Once-daily extended-release tacrolimus (LCPT) exhibits increased bioavailability versus immediate-release (IR-TAC) and prolonged release (PR-TAC) tacrolimus. Improvements in tremor were previously reported in a limited number of kidney transplant patients who switched to LCPT. We conducted a non-interventional, non-randomized, uncontrolled, longitudinal, prospective, multicenter study to assess the impact of switching to LCPT on tremor and quality of life (QoL) in a larger population of stable kidney transplant patients. The primary endpoint was change in The Essential Tremor Rating Assessment Scale (TETRAS) score; secondary endpoints included 12-item Short Form Survey (SF-12) scores, tacrolimus trough concentrations, neurologic symptoms, and safety assessments. Subgroup analyses were conducted to assess change in TETRAS score and tacrolimus trough concentration/dose (C0/D) ratio by prior tacrolimus formulation and tacrolimus metabolizer status. Among 221 patients, the mean decrease of TETRAS score after switch to LCPT was statistically significant (p < 0.0001 vs. baseline). There was no statistically significant difference in change in TETRAS score after switch to LCPT between patients who had received IR-TAC and those who had received PR-TAC before switch, or between fast and slow metabolizers of tacrolimus. The overall increase of C0/D ratio post-switch to LCPT was statistically significant (p < 0.0001) and from baseline to either M1 or M3 (both p < 0.0001) in the mITT population and in all subgroups. In the fast metabolizers group, the C0/D ratio crossed over the threshold of 1.05 ng/mL/mg after the switch to LCPT. Other neurologic symptoms tended to improve, and the SF-12 mental component summary score improved significantly. No new safety concerns were evident. In this observational study, all patients had a significant improvement of tremor, QoL and C0/D ratio post-switch to LCPT irrespective of the previous tacrolimus formulation administered (IR-TAC or PR-TAC) and irrespective from their metabolism status (fast or slow metabolizers).
Keywords: C0/D ratio; LCPT; extended-release tacrolimus; fast metabolizer; immunosuppression; kidney transplantation; quality of life; tremor.
Copyright © 2024 Giral, Grimbert, Morin, Bouvier, Buchler, Dantal, Garrigue, Bertrand, Kamar, Malvezzi, Moreau, Athea and Le Meur.
Conflict of interest statement
PG has received fees from BMS and Chiesi. YL has received speaker fees and consultant fees from AstraZeneca, Chiesi, and Hemarina. NK has received speaker fees and advisory board fees from Astellas, AstraZeneca, Biotest, CSL Behring, Chiesi, ExeViR, Hansa, Merck Sharp and Dohme, GlaxoSmithKline, Novartis Pharma, Sanofi, Sandoz, and Takeda. BM and YA work for Chiesi SAS. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Chiesi SAS France provided funding for this manuscript, and contributed to the study design; collection, analysis, and interpretation of the data; and the writing of the report. The decision to submit the report for publication was made by the authors.
Figures
References
-
- Agence de la Biomédecine. The Medical and Scientific Report 2021: Kidney Transplant (2021).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

