Anti-SARS-CoV-2 glyco-humanized polyclonal antibody XAV-19: phase II/III randomized placebo-controlled trial shows acceleration to recovery for mild to moderate patients with COVID-19
- PMID: 38694503
- PMCID: PMC11061480
- DOI: 10.3389/fimmu.2024.1330178
Anti-SARS-CoV-2 glyco-humanized polyclonal antibody XAV-19: phase II/III randomized placebo-controlled trial shows acceleration to recovery for mild to moderate patients with COVID-19
Abstract
Introduction: XAV-19 is a glyco-humanized swine polyclonal antibody targeting SARS-CoV-2 with high neutralizing activity. The safety and clinical efficacy of XAV-19 were investigated in patients with mild to moderate COVID-19.
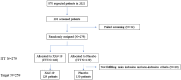
Methods: This phase II/III, multicentric, randomized, double-blind, placebo-controlled clinical trial was conducted to evaluate the safety and clinical efficacy of XAV-19 in patients with a seven-point WHO score of 2 to 4 at randomization, i.e., inpatients with COVID-19 requiring or not requiring low-flow oxygen therapy, and outpatients not requiring oxygen (EUROXAV trial, NCT04928430). Adult patients presenting in specialized or emergency units with confirmed COVID-19 and giving their consent to participate in the study were randomized to receive 150 mg of XAV-19 or placebo. The primary endpoint was the proportion of patients with aggravation within 8 days after treatment, defined as a worsening of the seven-point WHO score of at least one point between day 8 and day 1 (inclusion). The neutralization activity of XAV-19 against variants circulating during the trial was tested in parallel.
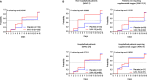
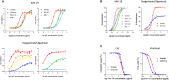
Results: From March 2021 to October 2022, 279 patients received either XAV-19 (N = 140) or placebo (N = 139). A slow enrollment and a low rate of events forced the termination of the premature trial. XAV-19 was well tolerated. Underpowered statistics did not allow the detection of any difference in the primary endpoint between the two groups or in stratified groups. Interestingly, analysis of the time to improvement (secondary endpoint) showed that XAV-19 significantly accelerated the recovery for patients with a WHO score of 2 or 3 (median at 7 days vs. 14 days, p = 0.0159), and even more for patients with a WHO score of 2 (4 days vs. 14 days, p = 0.0003). The neutralizing activity against Omicron and BA.2, BA.2.12.1, BA.4/5, and BQ.1.1 subvariants was shown.
Discussion: In this randomized placebo- controlled trial with premature termination, reduction of aggravation by XAV-19 at day 8 in patients with COVID-19 was not detectable. However, a significant reduction of the time to improvement for patients not requiring oxygen was observed. XAV-19 maintained a neutralizing activity against SARS-CoV-2 variants. Altogether, these data support a possible therapeutic interest for patients with mild to moderate COVID-19 requiring anti-SARS-CoV-2 neutralizing antibodies.
Clinical trial registration: https://clinicaltrials.gov/, identifier NCT04928430; https://www.clinicaltrialsregister.eu/about.html (EudraCT), identifier 2020-005979-12.
Keywords: (glyco-humanized) polyclonal antibody; COVID-19; SARS-CoV-2 variants; XAV-19; clinical trial.
Copyright © 2024 Poulakou, Royer, Evgeniev, Evanno, Shneiker, Marcelin, Vanhove, Duvaux, Marot and Calvez.
Conflict of interest statement
Authors P-JR, GE, FS, BV, and OD are employees of the company Xenothera. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

Similar articles
-
Evaluation of the safety and efficacy of XAV-19 in patients with COVID-19-induced moderate pneumonia: study protocol for a randomized, double-blinded, placebo-controlled phase 2 (2a and 2b) trial.Trials. 2021 Mar 9;22(1):199. doi: 10.1186/s13063-021-05132-9. Trials. 2021. PMID: 33750432 Free PMC article.
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
XAV-19, a Swine Glyco-Humanized Polyclonal Antibody Against SARS-CoV-2 Spike Receptor-Binding Domain, Targets Multiple Epitopes and Broadly Neutralizes Variants.Front Immunol. 2021 Nov 15;12:761250. doi: 10.3389/fimmu.2021.761250. eCollection 2021. Front Immunol. 2021. PMID: 34868003 Free PMC article.
-
Pharmacokinetics and Safety of XAV-19, a Swine Glyco-humanized Polyclonal Anti-SARS-CoV-2 Antibody, for COVID-19-Related Moderate Pneumonia: a Randomized, Double-Blind, Placebo-Controlled, Phase IIa Study.Antimicrob Agents Chemother. 2021 Aug 17;65(9):e0123721. doi: 10.1128/AAC.01237-21. Epub 2021 Aug 17. Antimicrob Agents Chemother. 2021. PMID: 34181475 Free PMC article. Clinical Trial.
-
Sotrovimab: A Review of Its Efficacy against SARS-CoV-2 Variants.Viruses. 2024 Jan 31;16(2):217. doi: 10.3390/v16020217. Viruses. 2024. PMID: 38399991 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

