Efficacy and safety of different immunotherapies combined with chemotherapy as first-line therapy in patients with small cell lung cancer: a network meta-analysis
- PMID: 38694505
- PMCID: PMC11061408
- DOI: 10.3389/fimmu.2024.1362537
Efficacy and safety of different immunotherapies combined with chemotherapy as first-line therapy in patients with small cell lung cancer: a network meta-analysis
Abstract
Background: The efficacy and safety of different immunosuppressants combined with chemotherapy in treating patients with small-cell lung cancer (extensive-disease small-cell lung cancer, limited-disease small-cell lung cancer and relapsed small-cell lung cancer) are still unknown, and there are no reports directly comparing the efficacy and safety of other immunotherapies.
Objective: This study aimed to compare the efficacy and safety of first-line immunotherapy combined with chemotherapy in patients with small-cell lung cancer.
Method: We searched Pubmed, Embase, Cochrane Library, CNKI, and Wanfang databases for relevant articles published from inception to November 11, 2020. The risk of bias of the included studies was conducted using the Cochrane risk-of-bias (RoB) tool. Multiple Bayesian network meta-analyses were performed. They conducted data analysis using R Studio and STATA version 15.1. The outcomes comprised overall survival (OS), progression-free survival (PFS), stability of response (SOR), duration of response (DOR) and adverse events of grade 3 or higher (AE grade≥3). A 95% confidence interval (CI) was provided for each estimate.
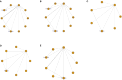
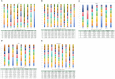
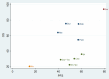
Results: This meta-analysis included 16 RCT studies with 5898 patients. For OS, relative to chemotherapy (MD=-4.49; 95%CI [-7.97, -1.03]), durvalumab plus tremelimumab (MD=-4.62; 95%CI [-9.08, -0.11]), ipilimumab (MD=-4.26; 95%CI [-8.01, -0.3]) and nivolumab(MD=-5.66; 95%CI [-10.44, -1.11]) and nivolumab plus ipilimumab (MD=-4.56; 95%CI [-8.7, -0.1]), serplulimab can significantly increase the OS of SCLC patients. There was no significant difference between PFS, SOR and DOR. Analysis of AE showed that different immunotherapy combined chemotherapy regimens were similar to single chemotherapy regarding the overall incidence of AE grade≥3. However, after the cumulative ranking of the common symptoms of different adverse reactions, it was found that nivolumab ranked first in the occurrence probability of anemia (99.08%), fatigue (84.78%), and decreased appetite (89.66%). durvalumab was the most likely in nausea (75.4%). Pembrolizumab (76.24%) was most likely to cause pruritus. Chemotherapy combined with immunotherapy caused less diarrhea than chemotherapy alone (80.16%).
Conclusions: According to our analysis, serplulimab combined with chemotherapy is more likely to show better efficacy with a manageable safety profile for small-cell lung cancer. However, the evidence for this comparison shows some limitations due to the number of literature.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42023486053.
Keywords: chemotherapy; immunotherapies; network meta-analysis; serplulimab; small-cell lung cancer.
Copyright © 2024 Gong, Li, Yu and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Efficacy and safety of first-line immunotherapy plus chemotherapy in treating patients with extensive-stage small cell lung cancer: a Bayesian network meta-analysis.Front Immunol. 2023 Jun 26;14:1197044. doi: 10.3389/fimmu.2023.1197044. eCollection 2023. Front Immunol. 2023. PMID: 37435087 Free PMC article.
-
Efficacy and safety of immune checkpoint inhibitors as neoadjuvant therapy in perioperative patients with non-small cell lung cancer: a network meta-analysis and systematic review based on randomized controlled trials.Front Immunol. 2024 Oct 1;15:1432813. doi: 10.3389/fimmu.2024.1432813. eCollection 2024. Front Immunol. 2024. PMID: 39416776 Free PMC article.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Comparison of efficacy and safety of PD-1/PD-L1 combination therapy in first-line treatment of advanced NSCLC: an updated systematic review and network meta-analysis.Clin Transl Oncol. 2024 Oct;26(10):2488-2502. doi: 10.1007/s12094-024-03442-3. Epub 2024 Apr 16. Clin Transl Oncol. 2024. PMID: 38625495
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
Cited by
-
Efficacy and side effects of pembrolizumab plus chemotherapy vs. chemotherapy alone in patients with advanced gastric or gastroesophageal junction adenocarcinoma: A meta‑analysis.Oncol Lett. 2024 Jun 12;28(2):371. doi: 10.3892/ol.2024.14504. eCollection 2024 Aug. Oncol Lett. 2024. PMID: 38910906 Free PMC article.
References
-
- García-Campelo R, Sullivan I, Arriola E, Insa A, Juan Vidal O, Cruz-Castellanos P, et al. . Correction to: SEOM-GECP Clinical guidelines for diagnosis, treatment and follow-up of small-cell lung cancer (SCLC) (2022). Clin Trans Oncol. (2023) 25:2760–2. doi: 10.1007/s12094-023-03290-7 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

