The ion channel TRPV5 regulates B-cell signaling and activation
- PMID: 38694510
- PMCID: PMC11061418
- DOI: 10.3389/fimmu.2024.1386719
The ion channel TRPV5 regulates B-cell signaling and activation
Abstract
Introduction: B-cell activation triggers the release of endoplasmic reticulum calcium stores through the store-operated calcium entry (SOCE) pathway resulting in calcium influx by calcium release-activated calcium (CRAC) channels on the plasma membrane. B-cell-specific murine knockouts of SOCE do not impact humoral immunity suggesting that alternative channels may be important.
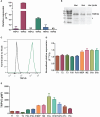
Methods: We identified a member of the calcium-permeable transient receptor potential (TRP) ion channel family, TRPV5, as a candidate channel expressed in B cells by a quantitative polymerase chain reaction (qPCR) screen. To further investigate the role of TRPV5 in B-cell responses, we generated a murine TRPV5 knockout (KO) by CRISPR-Cas9.
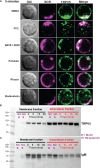
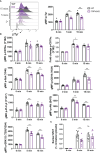
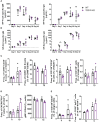
Results: We found TRPV5 polarized to B-cell receptor (BCR) clusters upon stimulation in a PI3K-RhoA-dependent manner. TRPV5 KO mice have normal B-cell development and mature B-cell numbers. Surprisingly, calcium influx upon BCR stimulation in primary TRPV5 KO B cells was not impaired; however, differential expression of other calcium-regulating proteins, such as ORAI1, may contribute to a compensatory mechanism for calcium signaling in these cells. We demonstrate that TRPV5 KO B cells have impaired spreading and contraction in response to membrane-bound antigen. Consistent with this, TRPV5 KO B cells have reduced BCR signaling measured through phospho-tyrosine residues. Lastly, we also found that TRPV5 is important for early T-dependent antigen specific responses post-immunization.
Discussion: Thus, our findings identify a role for TRPV5 in BCR signaling and B-cell activation.
Keywords: B cells; TRPV5; ion channels; signaling; transient receptor potential channel.
Copyright © 2024 Mahtani, Sheth, Smith, Benedict, Brecier, Ghasemlou and Treanor.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures




Similar articles
-
Regulation of the epithelial Ca²⁺ channel TRPV5 by reversible histidine phosphorylation mediated by NDPK-B and PHPT1.Mol Biol Cell. 2014 Apr;25(8):1244-50. doi: 10.1091/mbc.E13-04-0180. Epub 2014 Feb 12. Mol Biol Cell. 2014. PMID: 24523290 Free PMC article.
-
B-lymphocyte calcium influx.Immunol Rev. 2009 Sep;231(1):265-77. doi: 10.1111/j.1600-065X.2009.00822.x. Immunol Rev. 2009. PMID: 19754903 Free PMC article. Review.
-
Role of the transient receptor potential vanilloid 5 (TRPV5) protein N terminus in channel activity, tetramerization, and trafficking.J Biol Chem. 2011 Sep 16;286(37):32132-9. doi: 10.1074/jbc.M111.226878. Epub 2011 Jul 27. J Biol Chem. 2011. PMID: 21795703 Free PMC article.
-
β1-Adrenergic receptor signaling activates the epithelial calcium channel, transient receptor potential vanilloid type 5 (TRPV5), via the protein kinase A pathway.J Biol Chem. 2014 Jun 27;289(26):18489-96. doi: 10.1074/jbc.M113.491274. Epub 2014 May 14. J Biol Chem. 2014. PMID: 24828496 Free PMC article.
-
[Store-operated Calcium Entry into B Cells Regulates Autoimmune Inflammation].Yakugaku Zasshi. 2016;136(3):473-8. doi: 10.1248/yakushi.15-00246-3. Yakugaku Zasshi. 2016. PMID: 26935089 Review. Japanese.
Cited by
-
Both sides now: evolutionary traits of antigens and B cells in tolerance and activation.Front Immunol. 2024 Aug 9;15:1456220. doi: 10.3389/fimmu.2024.1456220. eCollection 2024. Front Immunol. 2024. PMID: 39185403 Free PMC article. Review.
-
PHPT1 acts as an inhibitor in high-altitude pulmonary hypertension via negative TRPV5 signaling regulation.J Transl Med. 2025 Aug 28;23(1):968. doi: 10.1186/s12967-025-06980-8. J Transl Med. 2025. PMID: 40877955 Free PMC article.
-
Mesenchymal stem cells in treating human diseases: molecular mechanisms and clinical studies.Signal Transduct Target Ther. 2025 Aug 22;10(1):262. doi: 10.1038/s41392-025-02313-9. Signal Transduct Target Ther. 2025. PMID: 40841367 Free PMC article. Review.
-
Dynamic Changes in Ion Channels during Myocardial Infarction and Therapeutic Challenges.Int J Mol Sci. 2024 Jun 12;25(12):6467. doi: 10.3390/ijms25126467. Int J Mol Sci. 2024. PMID: 38928173 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

