Cyclic AMP signaling promotes regeneration of cochlear synapses after excitotoxic or noise trauma
- PMID: 38694536
- PMCID: PMC11061447
- DOI: 10.3389/fncel.2024.1363219
Cyclic AMP signaling promotes regeneration of cochlear synapses after excitotoxic or noise trauma
Abstract
Introduction: Cochlear afferent synapses connecting inner hair cells to spiral ganglion neurons are susceptible to excitotoxic trauma on exposure to loud sound, resulting in a noise-induced cochlear synaptopathy (NICS). Here we assessed the ability of cyclic AMP-dependent protein kinase (PKA) signaling to promote cochlear synapse regeneration, inferred from its ability to promote axon regeneration in axotomized CNS neurons, another system refractory to regeneration.
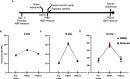
Methods: We mimicked NICS in vitro by applying a glutamate receptor agonist, kainic acid (KA) to organotypic cochlear explant cultures and experimentally manipulated cAMP signaling to determine whether PKA could promote synapse regeneration. We then delivered the cAMP phosphodiesterase inhibitor rolipram via implanted subcutaneous minipumps in noise-exposed CBA/CaJ mice to test the hypothesis that cAMP signaling could promote cochlear synapse regeneration in vivo.
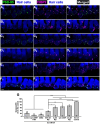
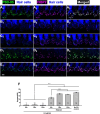
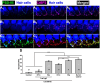
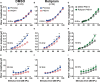
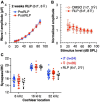
Results: We showed that the application of the cell membrane-permeable cAMP agonist 8-cpt-cAMP or the cAMP phosphodiesterase inhibitor rolipram promotes significant regeneration of synapses in vitro within twelve hours after their destruction by KA. This is independent of neurotrophin-3, which also promotes synapse regeneration. Moreover, of the two independent signaling effectors activated by cAMP - the cAMP Exchange Protein Activated by cAMP and the cAMP-dependent protein kinase - it is the latter that mediates synapse regeneration. Finally, we showed that systemic delivery of rolipram promotes synapse regeneration in vivo following NICS.
Discussion: In vitro experiments show that cAMP signaling promotes synapse regeneration after excitotoxic destruction of cochlear synapses and does so via PKA signaling. The cAMP phosphodiesterase inhibitor rolipram promotes synapse regeneration in vivo in noise-exposed mice. Systemic administration of rolipram or similar compounds appears to provide a minimally invasive therapeutic approach to reversing synaptopathy post-noise.
Keywords: auditory nerve; cochlea; cyclic AMP-dependent protein kinase; noise; regeneration; spiral ganglion neuron; synapse; synaptopathy.
Copyright © 2024 Hemachandran, Hu, Kane and Green.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

References
-
- Bandtlow C. E. (2003). Regeneration in the central nervous system. Exp. Gerontol. 38 79–86. - PubMed
-
- Bolger G., Michaeli T., Martins T., St John T., Steiner B., Rodgers L., et al. (1993). A family of human phosphodiesterases homologous to the dunce learning and memory gene product of Drosophila melanogaster are potential targets for antidepressant drugs. Mol. Cell Biol. 13 6558–6571. 10.1128/mcb.13.10.6558-6571.1993 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

