Retinoic acid modulation of granule cell activity and spatial discrimination in the adult hippocampus
- PMID: 38694537
- PMCID: PMC11061364
- DOI: 10.3389/fncel.2024.1379438
Retinoic acid modulation of granule cell activity and spatial discrimination in the adult hippocampus
Abstract
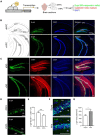
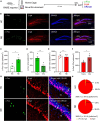
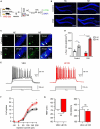
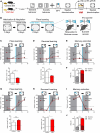
Retinoic acid (RA), derived from vitamin A (retinol), plays a crucial role in modulating neuroplasticity within the adult brain. Perturbations in RA signaling have been associated with memory impairments, underscoring the necessity to elucidate RA's influence on neuronal activity, particularly within the hippocampus. In this study, we investigated the cell type and sub-regional distribution of RA-responsive granule cells (GCs) in the mouse hippocampus and delineated their properties. We discovered that RA-responsive GCs tend to exhibit a muted response to environmental novelty, typically remaining inactive. Interestingly, chronic dietary depletion of RA leads to an abnormal increase in GC activation evoked by a novel environment, an effect that is replicated by the localized application of an RA receptor beta (RARβ) antagonist. Furthermore, our study shows that prolonged RA deficiency impairs spatial discrimination-a cognitive function reliant on the hippocampus-with such impairments being reversible with RA replenishment. In summary, our findings significantly contribute to a better understanding of RA's role in regulating adult hippocampal neuroplasticity and cognitive functions.
Keywords: dentate gyrus; granule cells; hippocampal neuroplasticity; retinoic acid; spatial discrimination; vitamin A.
Copyright © 2024 Yeo, Park, Kim, Rah, Shin, Oh, Jang, Lee, Yoon and Oh.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Basu R., Whitley S. K., Bhaumik S., Zindl C. L., Schoeb T. R., Benveniste E. N., et al. (2015). IL-1 signaling modulates activation of Stat transcription factors to antagonize retinoic acid signaling and control the TH 17 cell–iT reg cell balance. Nat. Immunol. 16 286–295. 10.1038/ni.3099 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

