Biotinylated Photocleavable Semiconductor Colloidal Quantum Dot Supraparticle Microlaser
- PMID: 38694721
- PMCID: PMC11059076
- DOI: 10.1021/acsanm.4c00668
Biotinylated Photocleavable Semiconductor Colloidal Quantum Dot Supraparticle Microlaser
Abstract
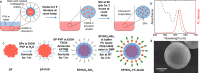
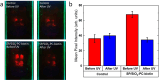
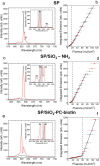
Luminescent supraparticles of colloidal semiconductor nanocrystals can act as microscopic lasers and are hugely attractive for biosensing, imaging, and drug delivery. However, biointerfacing these to increase functionality while retaining their main optical properties remains an unresolved challenge. Here, we propose and demonstrate red-emitting, silica-coated CdSxSe1-x/ZnS colloidal quantum dot supraparticles functionalized with a biotinylated photocleavable ligand. The success of each step of the synthesis is confirmed by scanning electron microscopy, energy dispersive X-ray and Fourier transform infrared spectroscopy, ζ-potential, and optical pumping measurements. The capture and release functionality of the supraparticle system is proven by binding to a neutravidin functionalized glass slide and subsequently cleaving off after UV-A irradiation. The biotinylated supraparticles still function as microlasers; e.g., a 9 μm diameter supraparticle has oscillating modes around 625 nm at a threshold of 58 mJ/cm2. This work is a first step toward using supraparticle lasers as enhanced labels for bionano applications.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
LinkOut - more resources
Full Text Sources
