Shooting area of infrared camera traps affects recorded taxonomic richness and abundance of ground-dwelling invertebrates
- PMID: 38694747
- PMCID: PMC11061542
- DOI: 10.1002/ece3.11357
Shooting area of infrared camera traps affects recorded taxonomic richness and abundance of ground-dwelling invertebrates
Abstract
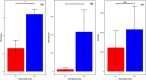
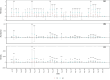
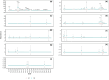
Ground-dwelling invertebrates are vital for soil biodiversity and function maintenance. Contemporary biodiversity assessment necessitates novel and automatic monitoring methods because of the threat of sharp reductions in soil biodiversity in farmlands worldwide. Using infrared camera traps (ICTs) is an effective method for assessing richness and abundance of ground-dwelling invertebrates. However, the influence that the shooting area of ICTs has on the diversity of ground-dwelling invertebrates has not been strongly considered during survey design. In this study, data from six ICTs with two shooting areas (A1, 38.48 cm2; A2, 400 cm2) were used to investigate ground-dwelling invertebrates in a farm in a city on the Eastern Coast of China from 20: 00 on July 31 to 00:00 on September 29, 2022. Over the course of 59 days and 1420 h, invertebrates within 9 taxa, 2447 individuals, and 112,909 ind./m2 were observed from 222,912 images. Our results show that ICTs with relatively large shooting areas recorded relatively high taxonomic richness and abundance of total ground-dwelling invertebrates, relatively high abundance of the dominant taxon, and relatively high daily and hourly abundance of most taxa. The shooting areas of ICTs significantly affected the recorded taxonomic richness and abundance of ground-dwelling invertebrates throughout the experimental period and at fine temporal resolutions. Overall, these results suggest that the shooting areas of ICTs should be considered when designing experiments, and ICTs with relatively large shooting areas are more favorable for monitoring the diversity of ground-dwelling invertebrates. This study further provides an automatic tool and high-quality data for biodiversity monitoring and protection in farmlands.
Keywords: Diplopoda; Formicidae; infrared camera trapping; sampling size; subtropical farmland.
© 2024 The Authors. Ecology and Evolution published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures








Similar articles
-
Daily dynamics of ground-dwelling invertebrate communities during and following an extreme high-temperature event in summer 2022, China.PLoS One. 2024 Aug 23;19(8):e0306823. doi: 10.1371/journal.pone.0306823. eCollection 2024. PLoS One. 2024. PMID: 39178186 Free PMC article.
-
Preliminary Study on Hourly Dynamics of a Ground-Dwelling Invertebrate Community in a Farmland Vineyard.Insects. 2024 Jan 2;15(1):27. doi: 10.3390/insects15010027. Insects. 2024. PMID: 38249034 Free PMC article.
-
Ground-dwelling invertebrate diversity in domestic gardens along a rural-urban gradient: Landscape characteristics are more important than garden characteristics.PLoS One. 2020 Oct 2;15(10):e0240061. doi: 10.1371/journal.pone.0240061. eCollection 2020. PLoS One. 2020. PMID: 33007013 Free PMC article.
-
A global meta-analysis reveals a consistent reduction of soil fauna abundance and richness as a consequence of land use conversion.Sci Total Environ. 2024 Oct 10;946:173822. doi: 10.1016/j.scitotenv.2024.173822. Epub 2024 Jun 19. Sci Total Environ. 2024. PMID: 38906293 Review.
-
Vertical stratification patterns of tropical forest vertebrates: a meta-analysis.Biol Rev Camb Philos Soc. 2023 Feb;98(1):99-114. doi: 10.1111/brv.12896. Epub 2022 Sep 8. Biol Rev Camb Philos Soc. 2023. PMID: 36073113 Review.
Cited by
-
Daily dynamics of ground-dwelling invertebrate communities during and following an extreme high-temperature event in summer 2022, China.PLoS One. 2024 Aug 23;19(8):e0306823. doi: 10.1371/journal.pone.0306823. eCollection 2024. PLoS One. 2024. PMID: 39178186 Free PMC article.
-
Ecological Insights From Camera Trapping Span Biological Taxa, and the Globe.Ecol Evol. 2025 Feb 2;15(2):e70947. doi: 10.1002/ece3.70947. eCollection 2025 Feb. Ecol Evol. 2025. PMID: 39901889 Free PMC article.
References
-
- Abensperg‐Traun, M. , & Steven, D. (1995). The effects of pitfall trap diameter on ant species richness (Hymenoptera: Formicidae) and species composition of the catch in a semi‐arid eucalypt woodland. Australian Journal of Ecology, 20, 282–287. 10.1111/j.1442-9993.1995.tb00540.x - DOI
-
- Amorim, F. W. , Marino, S. , Sanz‐Veiga, P. A. , Ollerton, J. , & Oliveira, P. E. (2022). Short flowers for long tongues: Functional specialization in a nocturnal pollination network of an asclepiad in long‐tongued hawkmoths. Biotropica, 54, 729–738. 10.1111/btp.13090 - DOI
-
- Brennan, K. E. C. , Majer, J. D. , & Moir, M. L. (2005). Refining sampling protocols for inventorying invertebrate biodiversity: Influence of drift‐fence length and pitfall trap diameter on spiders. Journal of Arachnology, 33, 681–702. 10.1636/M01-105.1 - DOI
-
- Brennan, K. E. C. , Majer, J. D. , & Reygaert, N. (1999). Determination of an optimal pitfall trap size for sampling spiders in a Western Australian jarrah forest. Journal of Insect Conservation, 3, 297–307. 10.1023/A:1009682527012 - DOI
Associated data
LinkOut - more resources
Full Text Sources

