Biomaterial-enhanced treg cell immunotherapy: A promising approach for transplant medicine and autoimmune disease treatment
- PMID: 38694761
- PMCID: PMC11061617
- DOI: 10.1016/j.bioactmat.2024.03.030
Biomaterial-enhanced treg cell immunotherapy: A promising approach for transplant medicine and autoimmune disease treatment
Abstract
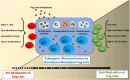
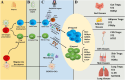
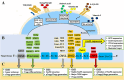
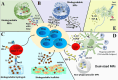
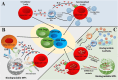
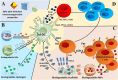
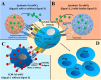
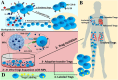
Regulatory T cells (Tregs) are crucial for preserving tolerance in the body, rendering Treg immunotherapy a promising treatment option for both organ transplants and autoimmune diseases. Presently, organ transplant recipients must undergo lifelong immunosuppression to prevent allograft rejection, while autoimmune disorders lack definitive cures. In the last years, there has been notable advancement in comprehending the biology of both antigen-specific and polyclonal Tregs. Clinical trials involving Tregs have demonstrated their safety and effectiveness. To maximize the efficacy of Treg immunotherapy, it is essential for these cells to migrate to specific target tissues, maintain stability within local organs, bolster their suppressive capabilities, and ensure their intended function's longevity. In pursuit of these goals, the utilization of biomaterials emerges as an attractive supportive strategy for Treg immunotherapy in addressing these challenges. As a result, the prospect of employing biomaterial-enhanced Treg immunotherapy holds tremendous promise as a treatment option for organ transplant recipients and individuals grappling with autoimmune diseases in the near future. This paper introduces strategies based on biomaterial-assisted Treg immunotherapy to enhance transplant medicine and autoimmune treatments.
Keywords: Autoimmune diseases; Biomaterials; Regulatory T cells; Transplantation.
© 2024 The Authors.
Conflict of interest statement
No declaration for G.B.K.
Figures
Similar articles
-
The Next Frontier of Regulatory T Cells: Promising Immunotherapy for Autoimmune Diseases and Organ Transplantations.Front Immunol. 2020 Sep 23;11:565518. doi: 10.3389/fimmu.2020.565518. eCollection 2020. Front Immunol. 2020. PMID: 33072105 Free PMC article. Review.
-
Challenges and opportunities in achieving effective regulatory T cell therapy in autoimmune liver disease.Semin Immunopathol. 2022 Jul;44(4):461-474. doi: 10.1007/s00281-022-00940-w. Epub 2022 May 31. Semin Immunopathol. 2022. PMID: 35641679 Free PMC article. Review.
-
Adoptive Cell Therapy with Tregs to Improve Transplant Outcomes: The Promise and the Stumbling Blocks.Curr Transplant Rep. 2016 Dec;3(4):265-274. doi: 10.1007/s40472-016-0114-9. Epub 2016 Oct 25. Curr Transplant Rep. 2016. PMID: 28529840 Free PMC article.
-
Therapy with regulatory T-cell infusion in autoimmune diseases and organ transplantation: A review of the strengths and limitations.Transpl Immunol. 2024 Aug;85:102069. doi: 10.1016/j.trim.2024.102069. Epub 2024 Jun 4. Transpl Immunol. 2024. PMID: 38844002 Review.
-
Chimeric Antigen Receptor (CAR) Treg: A Promising Approach to Inducing Immunological Tolerance.Front Immunol. 2018 Oct 12;9:2359. doi: 10.3389/fimmu.2018.02359. eCollection 2018. Front Immunol. 2018. PMID: 30369931 Free PMC article. Review.
Cited by
-
Special issue: Recent advances in immunotherapy and immunoengineering.Bioact Mater. 2025 Feb 28;48:529-530. doi: 10.1016/j.bioactmat.2025.01.038. eCollection 2025 Jun. Bioact Mater. 2025. PMID: 40104023 Free PMC article. No abstract available.
-
Advancements and challenges in stem cell transplantation for regenerative medicine.Heliyon. 2024 Aug 10;10(16):e35836. doi: 10.1016/j.heliyon.2024.e35836. eCollection 2024 Aug 30. Heliyon. 2024. PMID: 39247380 Free PMC article. Review.
References
-
- Sakaguchi S., et al. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995;155(3):1151–1164. - PubMed
-
- Friedman D.J., et al. The molecular Underpinnings of Treg plasticity in cancer. J. Immunol. 2023;210(1_Supplement):86.02.
-
- Bamidele A., et al. IL21 receptor-deficient regulatory T cells promote Resolution of intestinal inflammation by resisting metabolic Disturbance. J. Immunol. 2023;210(1_Supplement):66.06.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

