A scaffold vaccine to promote tumor antigen cross-presentation via sustained toll-like receptor-2 (TLR2) activation
- PMID: 38694764
- PMCID: PMC11061615
- DOI: 10.1016/j.bioactmat.2024.03.035
A scaffold vaccine to promote tumor antigen cross-presentation via sustained toll-like receptor-2 (TLR2) activation
Abstract
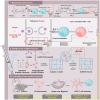
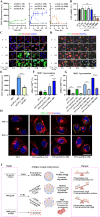
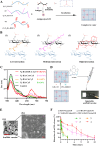
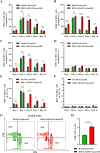
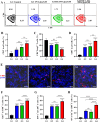
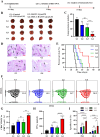
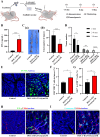
Cancer vaccination holds great promise for cancer treatment, but its effectiveness is hindered by suboptimal activation of CD8+ cytotoxic T lymphocytes, which are potent effectors to mediate anti-tumor immune responses. A possible solution is to switch antigen-presenting cells to present tumor antigens via the major histocompatibility complex class I (MHC-I) to CD8+ T cells - a process known as cross-presentation. To achieve this goal, we develop a three-dimensional (3D) scaffold vaccine to promote antigen cross-presentation by persisted toll-like receptor-2 (TLR2) activation after one injection. This vaccine comprises polysaccharide frameworks that "hook" TLR2 agonist (acGM) via tunable hydrophobic interactions and forms a 3D macroporous scaffold via click chemistry upon subcutaneous injection. Its retention-and-release of acGM enables sustained TLR2 activation in abundantly recruited dendritic cells in situ, inducing intracellular production of reactive oxygen species (ROS) in optimal kinetics that crucially promotes efficient antigen cross-presentation. The scaffold loaded with model antigen ovalbumin (OVA) or tumor specific antigen can generate potent immune responses against lung metastasis in B16-OVA-innoculated wild-type mice or spontaneous colorectal cancer in transgenic ApcMin/+ mice, respectively. Notably, it requires neither additional adjuvants nor external stimulation to function and can be adjusted to accommodate different antigens. The developed scaffold vaccine may represent a new, competent tool for next-generation personalized cancer vaccination.
Keywords: Cross-presentation; Polysaccharide; Reactive oxygen species; Scaffold vaccine; TLR2 agonist.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials

