The efficacy and safety of local 5-aminolevulinic acid-based photodynamic therapy in the treatment of cervical high-grade squamous intraepithelial lesion: a single center retrospective observational study
- PMID: 38694787
- PMCID: PMC11062129
- DOI: 10.3389/fonc.2024.1390982
The efficacy and safety of local 5-aminolevulinic acid-based photodynamic therapy in the treatment of cervical high-grade squamous intraepithelial lesion: a single center retrospective observational study
Abstract
Background: Typical treatments for cervical high-grade squamous intraepithelial lesion (HSIL) are invasive procedures. However, these procedures often come with several severe side effects, despite their positive effects on cervical HSIL. 5-aminolevulinic acid photodynamic therapy (ALA-PDT) is a non-invasive treatment that has been successfully used to treat cervical low-grade squamous intraepithelial lesion (LSIL). In this study, we aimed to further investigate the clinical efficacy and safety of ALA-PDT in the treatment of patients with cervical HSIL.
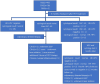
Methods: A total of 40 patients aged 20 - 41 years with cervical HSIL and high-risk Human Papilloma Virus (HR-HPV) infections were enrolled in this retrospective study from January 2019 to December 2022. Patients were treated with six times of ALA-PDT at intervals of 7-14 days. Three months after the treatment, the efficacy was evaluated through HPV genotyping and cervical cytology examination. If the cytological result was worse than ASC -US, the patient underwent colposcopy-directed biopsy immediately. Otherwise, patients would receive rigorous follow-up observation.
Results: Three months after receiving ALA-PDT treatment, 65% (26/40) of cervical HSIL patients at our center showed complete regression (cytological result: normal; HR-HPV: negative). This rate increased to 82.5% (33/40) at the 12-month follow-up. None of the patients experienced disease progression after ALA-PDT therapy. The risk of persistent HR-HPV infection was 32.5% (13/40) at the 3-month follow-up after ALA-PDT. Multivariate analyses identified cervical canal involvement as an independent risk factor for persistent HR-HPV infection at the 3-month follow-up after ALA-PDT treatment. During the treatment of the 40 patients with ALA-PDT, there were no reports of severe adverse reactions. Only a limited number of patients experienced slight discomfort symptoms.
Conclusion: ALA-PDT is safe and effective noninvasive therapy for patients with cervical HSIL and HR-HPV infections. It is particularly suitable for young women, who have been confirmed with cervical HSIL and have demand for fertility protection. Three months after ALA-PDT treatment, if a patient still has either ASC-US cervical cytological result and/or HR-HPV infection, rigorous observation is considered safe for her. Cervical canal involvement is an independent risk factor for persistent HR-HPV infection at the 3-month follow-up after ALA-PDT treatment.
Keywords: 5-aminolevulinic acid; cervical cancer; cervical high-grade squamous intraepithelial lesion; high-risk HPV; photodynamic therapy.
Copyright © 2024 Qian, Wang, Wu, Lu, Sun and Xu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
The role of photodynamic therapy in treating LSIL in women with high-risk HPV: a literature review.Lasers Med Sci. 2025 May 22;40(1):237. doi: 10.1007/s10103-025-04480-1. Lasers Med Sci. 2025. PMID: 40402167 Review.
-
Efficacy of photodynamic therapy with 5-aminolevulinic acid for the treatment of cervical high-grade squamous intraepithelial lesions with high-risk HPV infection: A retrospective study.Photodiagnosis Photodyn Ther. 2022 Dec;40:103068. doi: 10.1016/j.pdpdt.2022.103068. Epub 2022 Aug 21. Photodiagnosis Photodyn Ther. 2022. PMID: 36002107
-
Therapeutic effects of topical photodynamic therapy with 5-aminolevulinic acid on cervical high-grade squamous intraepithelial lesions.Photodiagnosis Photodyn Ther. 2022 Sep;39:102884. doi: 10.1016/j.pdpdt.2022.102884. Epub 2022 Apr 29. Photodiagnosis Photodyn Ther. 2022. PMID: 35500740
-
A prospective study of photodynamic therapy for cervical squamous intraepithelial lesion.Photodiagnosis Photodyn Ther. 2021 Jun;34:102185. doi: 10.1016/j.pdpdt.2021.102185. Epub 2021 Jan 14. Photodiagnosis Photodyn Ther. 2021. PMID: 33454394
-
Cervical Cancer Screening: A Review.JAMA. 2023 Aug 8;330(6):547-558. doi: 10.1001/jama.2023.13174. JAMA. 2023. PMID: 37552298 Review.
Cited by
-
The role of photodynamic therapy in treating LSIL in women with high-risk HPV: a literature review.Lasers Med Sci. 2025 May 22;40(1):237. doi: 10.1007/s10103-025-04480-1. Lasers Med Sci. 2025. PMID: 40402167 Review.
References
-
- Bingfeng H, Rongshou Z, Hongmei Z, Shaoming W, Kexin S, Ru C, et al. . Cancer incidence and mortality in China, 2022. J Natl Cancer Center. (2024), S2667005424000061. doi: 10.1016/j.jncc.2024.01.006 - DOI
-
- McCredie MR, Sharples KJ, Paul C, Baranyai J, Medley G, Jones RW, et al. . Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol. (2008) 9:425–34. doi: 10.1016/S1470-2045(08)70103-7 - DOI - PubMed
-
- Kyrgiou M, Athanasiou A, Paraskevaidi M, Mitra A, Kalliala I, Martin-Hirsch P, et al. . ”Adverse obstetric outcomes after local treatment for cervical preinvasive and early invasive disease according to cone depth: systematic review and meta-analysis. BMJ. (2016) 354:i3633. doi: 10.1136/bmj.i3633 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

