Succinylation of Park7 activates a protective metabolic response to acute kidney injury
- PMID: 38695076
- PMCID: PMC12151498
- DOI: 10.1152/ajprenal.00062.2024
Succinylation of Park7 activates a protective metabolic response to acute kidney injury
Abstract
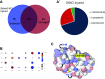
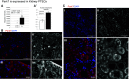
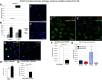
Acute kidney injury (AKI) is extremely prevalent among hospitalizations and presents a significant risk for the development of chronic kidney disease and increased mortality. Ischemia caused by shock, trauma, and transplant are common causes of AKI. To attenuate ischemic AKI therapeutically, we need a better understanding of the physiological and cellular mechanisms underlying damage. Instances of ischemia are most damaging in proximal tubule epithelial cells (PTECs) where hypoxic signaling cascades, and perhaps more rapidly, posttranslational modifications (PTMs), act in concert to change cellular metabolism. Here, we focus on the effects of the understudied PTM, lysine succinylation. We have previously shown a protective effect of protein hypersuccinylation on PTECs after depletion of the desuccinylase sirtuin5. General trends in the results suggested that hypersuccinylation led to upregulation of peroxisomal activity and was protective against kidney injury. Included in the list of changes was the Parkinson's-related deglycase Park7. There is little known about any links between peroxisome activity and Park7. In this study, we show in vitro and in vivo that Park7 has a crucial role in protection from AKI and upregulated peroxisome activity. These data in combination with published results of Park7's protective role in cardiovascular damage and chronic kidney disease lead us to hypothesize that succinylation of Park7 may ameliorate oxidative damage resulting from AKI and prevent disease progression. This novel mechanism provides a potential therapeutic mechanism that can be targeted.NEW & NOTEWORTHY Succinylation is an understudied posttranslational modification that has been shown to increase peroxisomal activity. Furthermore, increased peroxisomal activity has been shown to reduce oxidative stress and protect proximal tubules after acute kidney injury. Analysis of mass spectrometry succinylomic and proteomic data reveals a novel role for Parkinson's related Park7 in mediating Nrf2 antioxidant response after kidney injury. This novel protection pathway provides new insights for kidney injury prevention and development of novel therapeutics.
Keywords: Nrf2; Park7; acute kidney injury; sirtuin5; succinylation.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the author(s).
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

