Deficiency of the Deubiquitinase UCHL1 Attenuates Pulmonary Arterial Hypertension
- PMID: 38695173
- PMCID: PMC11262989
- DOI: 10.1161/CIRCULATIONAHA.123.065304
Deficiency of the Deubiquitinase UCHL1 Attenuates Pulmonary Arterial Hypertension
Abstract
Background: The ubiquitin-proteasome system regulates protein degradation and the development of pulmonary arterial hypertension (PAH), but knowledge about the role of deubiquitinating enzymes in this process is limited. UCHL1 (ubiquitin carboxyl-terminal hydrolase 1), a deubiquitinase, has been shown to reduce AKT1 (AKT serine/threonine kinase 1) degradation, resulting in higher levels. Given that AKT1 is pathological in pulmonary hypertension, we hypothesized that UCHL1 deficiency attenuates PAH development by means of reductions in AKT1.
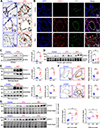
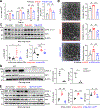
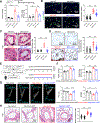
Methods: Tissues from animal pulmonary hypertension models as well as human pulmonary artery endothelial cells from patients with PAH exhibited increased vascular UCHL1 staining and protein expression. Exposure to LDN57444, a UCHL1-specific inhibitor, reduced human pulmonary artery endothelial cell and smooth muscle cell proliferation. Across 3 preclinical PAH models, LDN57444-exposed animals, Uchl1 knockout rats (Uchl1-/-), and conditional Uchl1 knockout mice (Tie2Cre-Uchl1fl/fl) demonstrated reduced right ventricular hypertrophy, right ventricular systolic pressures, and obliterative vascular remodeling. Lungs and pulmonary artery endothelial cells isolated from Uchl1-/- animals exhibited reduced total and activated Akt with increased ubiquitinated Akt levels. UCHL1-silenced human pulmonary artery endothelial cells displayed reduced lysine(K)63-linked and increased K48-linked AKT1 levels.
Results: Supporting experimental data, we found that rs9321, a variant in a GC-enriched region of the UCHL1 gene, is associated with reduced methylation (n=5133), increased UCHL1 gene expression in lungs (n=815), and reduced cardiac index in patients (n=796). In addition, Gadd45α (an established demethylating gene) knockout mice (Gadd45α-/-) exhibited reduced lung vascular UCHL1 and AKT1 expression along with attenuated hypoxic pulmonary hypertension.
Conclusions: Our findings suggest that UCHL1 deficiency results in PAH attenuation by means of reduced AKT1, highlighting a novel therapeutic pathway in PAH.
Keywords: models, animal; pulmonary arterial hypertension; ubiquitination.
Conflict of interest statement
None.
Figures



References
-
- Benza RL, Gomberg-Maitland M, Miller DP, Frost A, Frantz RP, Foreman AJ, Badesch DB and McGoon MD. The REVEAL Registry risk score calculator in patients newly diagnosed with pulmonary arterial hypertension. Chest. 2012;141:354–62. - PubMed
-
- McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, Rubin LJ, Tapson VF, Varga J, Harrington RA, Anderson JL, Bates ER, Bridges CR, Eisenberg MJ, Ferrari VA, Grines CL, Hlatky MA, Jacobs AK, Kaul S, Lichtenberg RC, Moliterno DJ, Mukherjee D, Pohost GM, Schofield RS, Shubrooks SJ, Stein JH, Tracy CM, Weitz HH and Wesley DJ. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation. 2009;119:2250–94. - PubMed
-
- Rhodes CJ, Batai K, Bleda M, Haimel M, Southgate L, Germain M, Pauciulo MW, Hadinnapola C, Aman J, Girerd B, Arora A, Knight J, Hanscombe KB, Karnes JH, Kaakinen M, Gall H, Ulrich A, Harbaum L, Cebola I, Ferrer J, Lutz K, Swietlik EM, Ahmad F, Amouyel P, Archer SL, Argula R, Austin ED, Badesch D, Bakshi S, Barnett C, Benza R, Bhatt N, Bogaard HJ, Burger CD, Chakinala M, Church C, Coghlan JG, Condliffe R, Corris PA, Danesino C, Debette S, Elliott CG, Elwing J, Eyries M, Fortin T, Franke A, Frantz RP, Frost A, Garcia JGN, Ghio S, Ghofrani HA, Gibbs JSR, Harley J, He H, Hill NS, Hirsch R, Houweling AC, Howard LS, Ivy D, Kiely DG, Klinger J, Kovacs G, Lahm T, Laudes M, Machado RD, Ross RVM, Marsolo K, Martin LJ, Moledina S, Montani D, Nathan SD, Newnham M, Olschewski A, Olschewski H, Oudiz RJ, Ouwehand WH, Peacock AJ, Pepke-Zaba J, Rehman Z, Robbins I, Roden DM, Rosenzweig EB, Saydain G, Scelsi L, Schilz R, Seeger W, Shaffer CM, Simms RW, Simon M, Sitbon O, Suntharalingam J, Tang H, Tchourbanov AY, Thenappan T, Torres F, Toshner MR, Treacy CM, Vonk Noordegraaf A, Waisfisz Q, Walsworth AK, Walter RE, Wharton J, White RJ, Wilt J, Wort SJ, Yung D, Lawrie A, Humbert M, Soubrier F, Tregouet DA, Prokopenko I, Kittles R, Graf S, Nichols WC, Trembath RC, Desai AA, Morrell NW, Wilkins MR, Consortium UNBRD, Consortium UPCS and Consortium UPB. Genetic determinants of risk in pulmonary arterial hypertension: international genome-wide association studies and meta-analysis. Lancet Respir Med. 2018. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

