Non-invasive high-frequency ventilation in newborn infants with respiratory distress
- PMID: 38695628
- PMCID: PMC11064768
- DOI: 10.1002/14651858.CD012712.pub2
Non-invasive high-frequency ventilation in newborn infants with respiratory distress
Abstract
Background: Respiratory distress occurs in up to 7% of newborns, with respiratory support (RS) provided invasively via an endotracheal (ET) tube or non-invasively via a nasal interface. Invasive ventilation increases the risk of lung injury and chronic lung disease (CLD). Using non-invasive strategies, with or without minimally invasive surfactant, may reduce the need for mechanical ventilation and the risk of lung damage in newborn infants with respiratory distress.
Objectives: To evaluate the benefits and harms of nasal high-frequency ventilation (nHFV) compared to invasive ventilation via an ET tube or other non-invasive ventilation methods on morbidity and mortality in preterm and term infants with or at risk of respiratory distress.
Search methods: We searched CENTRAL, MEDLINE, Embase, CINAHL and three trial registries in April 2023.
Selection criteria: Randomised controlled trials (RCTs), cluster- or quasi-RCTs of nHFV in newborn infants with respiratory distress compared to invasive or non-invasive ventilation.
Data collection and analysis: Two authors independently selected the trials for inclusion, extracted data, assessed the risk of bias, and undertook GRADE assessment.
Main results: We identified 33 studies, mostly in low- to middle-income settings, that investigated this therapy in 5068 preterm and 46 term infants. nHFV compared to invasive respiratory therapy for initial RS We are very uncertain whether nHFV reduces mortality before hospital discharge (RR 0.67, 95% CI 0.20 to 2.18; 1 study, 80 infants) or the incidence of CLD (RR 0.38, 95% CI 0.09 to 1.59; 2 studies, 180 infants), both very low-certainty. ET intubation, death or CLD, severe intraventricular haemorrhage (IVH) and neurodevelopmental disability (ND) were not reported. nHFV vs nasal continuous positive airway pressure (nCPAP) used for initial RS We are very uncertain whether nHFV reduces mortality before hospital discharge (RR 1.00, 95% CI 0.41 to 2.41; 4 studies, 531 infants; very low-certainty). nHFV may reduce ET intubation (RR 0.52, 95% CI 0.33 to 0.82; 5 studies, 571 infants), but there may be little or no difference in CLD (RR 1.35, 95% CI 0.80 to 2.27; 4 studies, 481 infants); death or CLD (RR 2.50, 95% CI 0.52 to 12.01; 1 study, 68 participants); or severe IVH (RR 1.17, 95% CI 0.36 to 3.78; 4 studies, 531 infants), all low-certainty evidence. ND was not reported. nHFV vs nasal intermittent positive-pressure ventilation (nIPPV) used for initial RS nHFV may result in little to no difference in mortality before hospital discharge (RR 1.86, 95% CI 0.90 to 3.83; 2 studies, 84 infants; low-certainty). nHFV may have little or no effect in reducing ET intubation (RR 1.33, 95% CI 0.76 to 2.34; 5 studies, 228 infants; low-certainty). There may be a reduction in CLD (RR 0.63, 95% CI 0.42 to 0.95; 5 studies, 307 infants; low-certainty). A single study (36 infants) reported no events for severe IVH. Death or CLD and ND were not reported. nHFV vs high-flow nasal cannula (HFNC) used for initial RS We are very uncertain whether nHFV reduces ET intubation (RR 2.94, 95% CI 0.65 to 13.27; 1 study, 37 infants) or reduces CLD (RR 1.18, 95% CI 0.46 to 2.98; 1 study, 37 participants), both very low-certainty. There were no mortality events before hospital discharge or severe IVH. Other deaths, CLD and ND, were not reported. nHFV vs nCPAP used for RS following planned extubation nHFV probably results in little or no difference in mortality before hospital discharge (RR 0.92, 95% CI 0.52 to 1.64; 6 studies, 1472 infants; moderate-certainty). nHFV may result in a reduction in ET reintubation (RR 0.42, 95% CI 0.35 to 0.51; 11 studies, 1897 infants) and CLD (RR 0.78, 95% CI 0.67 to 0.91; 10 studies, 1829 infants), both low-certainty. nHFV probably has little or no effect on death or CLD (RR 0.90, 95% CI 0.77 to 1.06; 2 studies, 966 infants) and severe IVH (RR 0.80, 95% CI 0.57 to 1.13; 3 studies, 1117 infants), both moderate-certainty. We are very uncertain whether nHFV reduces ND (RR 0.92, 95% CI 0.37 to 2.29; 1 study, 74 infants; very low-certainty). nHFV versus nIPPV used for RS following planned extubation nHFV may have little or no effect on mortality before hospital discharge (RR 1.83, 95% CI 0.70 to 4.79; 2 studies, 984 infants; low-certainty). There is probably a reduction in ET reintubation (RR 0.69, 95% CI 0.54 to 0.89; 6 studies, 1364 infants), but little or no effect on CLD (RR 0.88, 95% CI 0.75 to 1.04; 4 studies, 1236 infants); death or CLD (RR 0.92, 95% CI 0.79 to 1.08; 3 studies, 1070 infants); or severe IVH (RR 0.78, 95% CI 0.55 to 1.10; 4 studies, 1162 infants), all moderate-certainty. One study reported there might be no difference in ND (RR 0.88, 95% CI 0.35 to 2.16; 1 study, 72 infants; low-certainty). nHFV versus nIPPV following initial non-invasive RS failure nHFV may have little or no effect on mortality before hospital discharge (RR 1.44, 95% CI 0.10 to 21.33); or ET intubation (RR 1.23, 95% CI 0.51 to 2.98); or CLD (RR 1.01, 95% CI 0.70 to 1.47); or severe IVH (RR 0.47, 95% CI 0.02 to 10.87); 1 study, 39 participants, all low- or very low-certainty. Other deaths or CLD and ND were not reported.
Authors' conclusions: For initial RS, we are very uncertain if using nHFV compared to invasive respiratory therapy affects clinical outcomes. However, nHFV may reduce intubation when compared to nCPAP. For planned extubation, nHFV may reduce the risk of reintubation compared to nCPAP and nIPPV. nHFV may reduce the risk of CLD when compared to nCPAP. Following initial non-invasive respiratory support failure, nHFV when compared to nIPPV may result in little to no difference in intubation. Large trials, particularly in high-income settings, are needed to determine the role of nHFV in initial RS and following the failure of other non-invasive respiratory support. Also, the optimal settings of nHVF require further investigation.
Copyright © 2024 The Authors. Cochrane Database of Systematic Reviews published by John Wiley & Sons, Ltd. on behalf of The Cochrane Collaboration.
Conflict of interest statement
Mohamed E Abdel‐Latif is an Associate Editor with the Cochrane Neonatal Group, but was not involved in the editorial acceptance or assessment of this review.
Olive Tan does not have any interests to disclose at this time.
Michelle Fiander is an Information Specialist and Managing Editor with the Cochrane Neonatal Group, but was not involved in the editorial acceptance or assessment of this review.
David A Osborn is a Senior Editor with the Cochrane Neonatal Group, but was not involved in the editorial acceptance or assessment of this review.
Figures
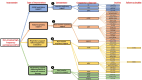
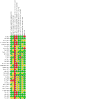


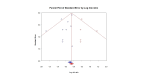



























































































Update of
- doi: 10.1002/14651858.CD012712
References
References to studies included in this review
Ali 2023 {published and unpublished data}
-
- ACTRN12622000291785. Efficacy of nasal high-frequency oscillation ventilation (NHFOV) compared to noninvasive positive-pressure ventilation (NIPPV) in pre-terms with respiratory distress syndrome during the first 7 days of life. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12622000291785 (first received 16 February 2022). [CENTRAL: CN-02376417]
-
- Ali R, Mahmud S. Nasal high-frequency oscillatory ventilation versus nasal intermittent positive pressure ventilation In pre-terms with respiratory distress syndrome during early neonatal period: a randomized controlled trial. Pakistan Armed Forces Medical Journal 2023;73(1):151-4. [DOI: 10.51253/pafmj.v73i1.8186] - DOI
Chen 2019 {published data only}
-
- NCT03140891. Nasal high frequency oscillation ventilation (NHFOV) for respiratory distress syndrome [Nasal high frequency oscillation ventilation(NHFOV) vs. nasal continuous positive airway pressure(NCPAP) as post‐extubation respiratory support in preterm infants with respiratory distress syndrome: a randomized controlled trial]. clinicaltrials.gov/study/NCT03140891 (first received 04 May 2017). [CENTRAL: CN-01493871]
Cheng 2021 {published data only}
-
- Wei C, Shuanggen M. Clinical application of non-invasive high-frequency oscillation ventilation in preterm infants with respiratory distress syndrome: a randomized controlled trial [无创高频振荡通气在早产儿呼吸窘迫综合征中的应用]. Journal of Shenyang Medical College 2021;23(3):229-39. [DOI: 10.16753/j.enji.1008-2344.2021.03.010] - DOI
De La Roque 2011 {published data only}
-
- De La Roque ED, Bertrand C, Tandonnet O, Rebola M, Roquand E, Renesme L, et al. Nasal high frequency percussive ventilation versus nasal continuous positive airway pressure in transient tachypnea of the newborn: a pilot randomized controlled trial. Pediatric Pulmonology 2011;46(3):218-23. [DOI: 10.1002/ppul.21354] [PMID: ] - DOI - PubMed
-
- NCT00556738. Intrapulmonary percussive ventilation (IPV) versus nasal continuous positive airway pressure ventilation (nCPAP) in transient respiratory distress of the newborn (HFPV) [Intrapulmonary percussive ventilation and nasal continuous positive airway pressure ventilation in transient respiratory distress of the newborn: a randomized controlled trial]. clinicaltrials.gov/ct2/show/NCT00556738 (first received 27 November 2007). [CENTRAL: CN-02016385]
El Ashker 2022 {published data only (unpublished sought but not used)}PACTR202207646279984
-
- El Ashker GN, EL Sharkawy HM, El Basset Mohamed Abo, El Ezz AA, Ibrahim AM. Nasal high frequency oscillatory ventilation versus nasal continuous positive airway pressure as initial therapy in respiratory distress syndrome in preterm newborns. NeuroQuantology 2022;20(10):10255-64. [DOI: 10.14704/nq.2022.20.10.NQ55996] - DOI
-
- PACTR202207646279984. Nasal high frequency oscillatory ventilation versus nasal continuous positive airway pressure as initial therapy in respiratory distress syndrome in preterm newborns. trialsearch.who.int/Trial2.aspx?TrialID=PACTR202207646279984 (first received 14 July 2022). [CENTRAL: CN-02458295]
Feng 2019 {published data only}
-
- Aimin F, Xiuchun X, Miao W, Ying Y, Xiaying W, Xiaoxia G. Observation of the effect of pulmonary surfactant combined with non-invasive high-frequency oscillatory ventilation in the treatment of neonatal respiratory distress syndrome [肺表面活性物质联合无创高频振荡通气治疗新生儿呼吸窘迫综合征的效果观察]. Hebei Medicine 2019;25(03):551-5.
Fischer 2019 {published data only}
-
- NCT02340299. Nasal HFOV versus nasal CPAP to reduce post-extubation pCO2 [Nasal high frequency oscillation ventilation versus nasal continuous positive airway pressure to reduce post-extubation pCO2 in very low birth weight infants: a randomized controlled trial]. clinicaltrials.gov/show/NCT02340299 (first received 16 January 2015). [CENTRAL: CN-02039356]
Guo 2021 {published data only}
-
- Guo M, Qiwei W, Dingli L, Lei W. Application of non-invasive high-frequency oscillatory ventilation in the treatment of neonatal respiratory distress syndrome [无创高频震荡通气在新生儿呼吸窘迫综合征治疗中的应用]. Chinese Journal of General Practice 2021;19(9):1514-17; 1556. [DOI: 10.16766/j.cnki.issn.1674-4152.002100] - DOI
Iranpour 2019 {published data only}
-
- IRCT2017062734782N1. The efficacy of nasal high frequency ventilation in treatment of neonatal respiratory syndrome [Therapeutic effect of nasal high frequency ventilation(NHFV) versus nasal continuous positive airway pressure (NCPAP) for respiratory distress syndrome in newborn with birth with more than 1000 gram and more than 30 week of gestational age]. trialsearch.who.int/Trial2.aspx?TrialID=IRCT2017062734782N1 (first received 09 September 2017). [PMID: ]
-
- Iranpour R, Armanian AM, Abedi AR, Farajzadegan Z. Nasal high-frequency oscillatory ventilation (nHFOV) versus nasal continuous positive airway pressure (NCPAP) as an initial therapy for respiratory distress syndrome (RDS) in preterm and near-term infants. BMJ Paediatrics Open 2019;3(1):e000443. [DOI: 10.1136/bmjpo-2019-000443] [PMID: ] - DOI - PMC - PubMed
Jiang 2020 {published data only}
-
- Jiang S, Wang L, Peng C, Wu F, Qin T. A comparative study of two noninvasive positive pressure ventilation modes combined with curosurf in the treatment of respiratory distress syndrome in premature infants. International Journal of Clinical and Experimental Medicine 2020;13(2):981-7. [URL: e-century.us/files/ijcem/13/2/ijcem0101258.pdf]
Li 2019 {published data only}
-
- Li H, Xingwang Z, Wanjun W. Efficacy of non-invasive high frequency oscillatory ventilation as post-extubation respiratory support in preterm neonates: a randomized controlled trial [无创高频振荡通气在早产儿有创机械通气撤机后呼吸支持效果的随机对照研究]. Journal of Third Military Medical University 2019;41(17):1688-92. [DOI: 10.16016/j.1000-5404.201903161] - DOI
Li 2021 {published data only}
-
- ChiCTR1900024289. Non-invasive high-frequency oscillatory ventilation in preterm infants after extubation. trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR1900024289 (first received 5 July 2019). [CENTRAL: CN-01975039]
Lou 2017 {published data only}
-
- Lou WB, Zhang WX. Noninvasive high-frequency oscillatory ventilation versus nasal continuous positive airway pressure in premature infants with respiratory distress syndrome after weaning: a randomized controlled trial [经鼻无创高频振荡通气和持续气道正压通气在早产儿呼吸窘迫综合征撤机后的应用比较]. Guangdong Medical Journal 2017;38(13):2037-40.
Lou 2018 {published data only}
-
- Lou WB, Zhang WX, Yuan L, Zhang B. Comparative study of noninvasive high-frequency oscillatory ventilation and bilevel positive airway pressure ventilation for preterm infants with respiratory distress syndrome [无创高频振荡通气和双水平正压通气在早产儿呼吸窘迫综合征中的临床应用效果比较研究]. Chinese General Practice 2018;21(16):1983–8. [DOI: 10.3969/j.issn.1007-9572.2018.16.016] - DOI
Malakian 2020 {published data only}
-
- Malakian A, Bashirnezhadkhabaz S, Aramesh MR, Dehdashtian M. Noninvasive high-frequency oscillatory ventilation versus nasal continuous positive airway pressure in preterm infants with respiratory distress syndrome: a randomized controlled trial. Journal of Maternal-Fetal & Neonatal Medicine 2020;33(15):2601-7. [DOI: 10.1080/14767058.2018.1555810] [PMID: ] - DOI - PubMed
Menshykova 2015 {published data only}
-
- Menshykova AO, Dobryanskyy DO, Salabay ZV, Kuzminov YB. PS-381 Nasal high frequency ventilation is not more effective than noninvasive ventilation to prevent extubation failure in very preterm infants. The 5th Congress of the European Academy of Paediatric Societies EAPS October 2014 17–21 October, Barcelona, Spain. Archives of Disease in Childhood 2014;99(Suppl 2):A250. [DOI: 10.1136/archdischild-2014-307384.679] - DOI
-
- Menshykova AO, Dobryanskyy DO. Respiratory support of preterm infants after extubation: comparison of clinical efficacy of standard and high-frequency non-invasive ventilation [ДИХАЛЬНА ПІДТРИМКА НЕДОНОШЕНИХНОВОНАРОДЖЕНИХ ПІСЛЯ ЕКСТУБАЦІЇ:ПОРІВНЯННЯ КЛІНІЧНОЇ ЕФЕКТИВНОСТІСТАНДАРТНОЇ І ВИСОКОЧАСТОТНОЇНЕІНВАЗИВНОЇ ВЕНТИЛЯЦІЇ]. Neonatology, Surgery and Perinatal Medicine 2015;1(15):37-43. [DOI: 10.24061/2413-4260.V.1.15.2015.7] - DOI
Mukerji 2017 {published data only}
-
- Mukerji A, Sarmiento K, Lee B, Hassall K, Shah V. Non-invasive high frequency oscillatory ventilation (NIHFOV) versus bi-phasic continuous positive airway pressure (BP-CPAP) following CPAP failure in infants < 1,250 grams: a pilot randomized controlled trial. Journal of Perinatology 2017;37(1):49-53. [DOI: 10.1038/jp.2016.172] [PMID: ] - DOI - PubMed
-
- NCT02051491. Non-invasive ventilation for extubation success in infants less than 1,250 grams (NOVEL) [A randomized controlled trial of biphasic nasal continuous positive airway pressure (BP-NCPAP) vs. non-invasive high frequency ventilation (NIHFV) following NCPAP failure: a pilot study]. clinicaltrials.gov/ct2/history/NCT02051491 (first received 31 January 2013). [CENTRAL: CN-01543384]
Oktem 2021 {published data only}
Seth 2021 {published data only}
-
- CTRI/2019/07/020055. Comparing two methods of giving support to respiratory system of babies born early, following the removal of respiratory support which was being provided through a tube placed in babys airway [Nasal high frequency oscillatory ventilation (nHFOV) versus non invasive positive pressure ventilation (NIPPV) as a post extubation respiratory support in preterm neonates (26-37 weeks) admitted in a tertiary care centre. A randomized controlled trial]. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2019/07/020055 (first received 5 July 2019). [CENTRAL: CN-02065897]
Wang 2020 {published data only}
-
- Wang W. Evaluation of the effect of non-invasive high-frequency ventilation on respiratory support after weaning in neonates with acute respiratory distress syndrome [无创高频通气应用于新生儿急性呼吸窘迫综合征撤机后呼吸支持的效果评价]. Chinese Community Physician 2020;25:35-6.
Wang 2023 {published data only}
Xu 2020 {published data only}
-
- Xu S. Clinical study of non-invasive high frequency oscillatory ventilation combined with pulmonary surfactant in treatment of neonates with neonatal respiratory distress syndrome [无创高频震荡通气联合肺表面活性物质治疗新生儿呼吸窘迫综合征的临床研究]. Journal of Clinical Medicine in Practice 2020;24(8):29-32. [DOI: 10.7619/jcmp.202008008] - DOI
Yang 2020 {published data only}
-
- Yang H. Clinical effect of non-invasive high frequency oscillatory ventilation in the treatment of infants with neonatal respiratory distress syndrome [无创高频振荡通气治疗新生儿呼吸窘迫综合征患儿的临床疗效]. Guide of Chinese Medicine 2020;18(25):53-4. [URL: caod.oriprobe.com/articles/59972554/Clinical_Effect_of_Non_invasive_High...]
Yang 2021 {published data only}
-
- Yang X-Q. A randomized controlled study on the effect of non-invasive high-frequency oscillatory ventilation on respiratory support after invasive mechanical ventilation withdrawal in preterm infants [无创高频振荡通气在早产儿有创机械通气撤机后呼吸支持效果的随机对照研究]. China Medical Device Information 2019:74-5. [DOI: 10.15971/j.cnki.cmdi.2021.02.036] - DOI
Yuan 2021 {published data only}
-
- Yuan G, Liu H, Wu Z, Chen X. Comparison of the efficacy and safety of three non-invasive ventilation methods in the initial treatment of premature infants with respiratory distress syndrome. International Journal of Clinical and Experimental Medicine 2021;14(2):1065-76. [URL: ijcem.com/files/ijcem0116814.pdf]
Zhang 2021 {published data only}
-
- Zhang H, Fu H. Non-invasive high frequency oscillatory ventilation versus nasal continuous positive airway pressure as post-extubation respiratory support in preterm neonates [无创高频振荡通气和持续气道正压通气在早产儿拔管后的应用比较]. Journal of Hubei University of Medicine 2021;40(4):391-5. [DOI: 10.13819 / j.issn.2096-708X.2021.04.013]
Zhang 2022a {published data only}
-
- Zhang H. Efficacy of non-invasive high-frequency oscillatory ventilation in treatment of neonatal respiratory distress syndrome [无创高频震荡通气治疗新生儿 呼吸窘迫综合征的疗效观察]. Zhejiang Medicine 2022;44(04):404-10.
Zhang 2022b {published data only}
-
- Zhang R, Shen L, Wen J, Ye L, Liang Z, Han F. Application of non-invasive high frequency oscillation ventilation after extubation in persistent pulmonary hypertension of newborn [无创高频振荡通气在新生儿持续肺动脉高压拔管后的应用]. Shenzhen Journal of Integrated Traditional Chinese and Western Medicine 2022;32(1):101-3. [DOI: 10.16458/j.cnki.1007-0893.2022.01.032] - DOI
Zhenyu 2019 {published data only}
-
- Zhenyu L, Chen Na C, Wenjia W. Evaluation of the effect of non-invasive high-frequency ventilation on respiratory support after weaning in neonates with acute respiratory distress syndrome [无创高频通气应用于新生儿急性呼吸窘迫综合征撤机后呼吸支持的效果评价]. Chinese Medical Science 2019;9(18):110-2.
Zhu 2017 {published data only}
-
- NCT03099694. NHFOV vs. NCPAP as a primary treatment to neonatal respiratory distress syndrome (NRDS). clinicaltrials.gov/show/NCT03099694 (first received 4 April 2017). [CENTRAL: CN-01563084]
-
- Zhu X, Yan J, Ran Q, Gao Q, Liao C, Shi Y. A preliminary study of non-invasive high-frequency oscillatory ventilation in the treatment of neonatal respiratory distress syndrome. Chinese Journal of Neonatology 2017;32(4):291-4. [DOI: 10.3760/cma.j.issn.2096-2932.2017.04.012] [https://rs.yiigle.com/resource_static.jspx?contentId=999695] - DOI
-
- Zhu XW, Shi Y. Clinical settings in a preliminary study: noninvasive high-frequency oscillatory ventilation versus nasal continuous positive airway pressure in preterm infants with moderate-severe respiratory distress syndrome. Pediatric Pulmonology 2018;53(4):389-90. [DOI: 10.1002/ppul.2388] - DOI - PubMed
Zhu 2021 {published data only}
-
- NCT03099694. NHFOV vs. NCPAP as a primary treatment to neonatal respiratory distress syndrome (NRDS). clinicaltrials.gov/ct2/show/NCT03099694 (first registered 4 April 2017).
-
- Zhu XW, Shi Y, Shi LP, Liu L, Xue J, Ramanathan R. Non-invasive high-frequency oscillatory ventilation versus nasal continuous positive airway pressure in preterm infants with respiratory distress syndrome: study protocol for a multi-center prospective randomized controlled trial. Trials 2018;19(1):319. [DOI: 10.1186/s13063-018-2673-9] - DOI - PMC - PubMed
Zhu 2022 {published data only}
-
- Zhu X, Qi H, Feng Z, Shi Y, De Luca D. Nasal continuous positive airway pressure (NCPAP) vs noninvasive positive pressure ventilation (NIPPV) vs noninvasive high frequency oscillation ventilation (NHFOV) as post-extubation support in preterm neonates: a multi-center, randomised, assessor-blinded controlled trial. Available at SSRN: https://ssrn.com/abstract=3931757 (preprint Lancet) 27 Sep 2021;None:1-24. [DOI: ]
-
- Li H, Zhu X, Wang W. Efficacy of non-invasive high frequency oscillatory ventilation as post-extubation respiratory support in preterm neonates: a randomized controlled trial [无创高频振荡通气在早产儿有创机械通气撤机后呼吸支持效果的随机对照研究]. Journal of Third Military Medical University 2019;41(17):1688-92. [DOI: 10.16016/j.1000-5404.201903161] - DOI
-
- NCT03181958. A trial comparing noninvasive ventilation strategies in preterm infants following extubation. clinicaltrials.gov/show/NCT03181958 (first received 9 June 2017).
-
- Shi Y, De Luca D, for the NASal OscillatioN post-Extubation (NASONE) study group. Continuous positive airway pressure (CPAP) vs noninvasive positive pressure ventilation (NIPPV) vs noninvasive high frequency oscillation ventilation (NHFOV) as post-extubation support in preterm neonates: protocol for an assessor-blinded,multicenter, randomized controlled trial. BMC Pediatrics 2019;19(256):1-15. [DOI: 10.1186/s12887-019-1625-1] - DOI - PMC - PubMed
-
- Zhu X, Qi H, Feng Z, Shi Y, De Luca D, Nasal Oscillation Post-Extubation (NASONE) Study Group. Noninvasive high-frequency oscillatory ventilation vs nasal continuous positive airway pressure vs nasal intermittent positive pressure ventilation as post extubation support for preterm neonates in China: a randomized clinical trial. JAMA Pediatrics 2022;176(6):551-9. [DOI: 10.1001/jamapediatrics.2022.0710] - DOI - PMC - PubMed
Zou 2020 {published data only}
-
- Zou F, Tang W. Efficacy and safety of non-invasive high-frequency oscillatory ventilation in very low birth weight/extremely low birth weight infants with respiratory distress syndrome [无创高频通气治疗极低/超低出生体重儿呼吸窘迫综 合征的疗效及安全性研究]. Jiangxi Medicine 2020;55(12):1777-80. [DOI: 10.3969/j.issn.1006-2238.2020.12.010] - DOI
References to studies excluded from this review
Aktas 2016 {published data only}
Ali 2020 {published data only}
Al Tawil 2011 {published data only}
-
- Al Tawil KI, Ahmed IA, Tawakol H, Bin Saleem N, AlSaif SA, Eldemerdash A. Management of pulmonary interstitial emphysema in a premature infant using nasopharyngeal high-frequency oscillatory ventilation. Journal of Pulmonary & Respiratory Medicine 2011;1(3):1000108. [DOI: 10.4172/2161-105X.1000108] [URL: www.hilarispublisher.com/open-access/management-of-emphysema-in-a-premat...] - DOI
Bottino 2018 {published data only}
-
- NCT02772835. nHFOV vs nCPAP: effects on gas exchange for the treatment of neonates recovering from RDS [Nasal HFOV vs nasal CPAP: effects on gas exchange for the treatment of neonates recovering from respiratory distress syndrome. A multicenter randomized controlled trial]. clinicaltrials.gov/show/NCT02772835 (first received 16 May 2016). [CENTRAL: CN-02039635]
Cao 2020 {published data only}
Colaizy 2008 {published data only}
-
- Colaizy TT, Younis UMM, Bell EF, Klein JM. Nasal high-frequency ventilation for premature infants. Acta Paediatrica 2008;97(11):1518-22. [DOI: 10.1111/j.1651-2227.2008.00900.x] [PMID: ] [URL: www.ncbi.nlm.nih.gov/pmc/articles/PMC3976963/] - DOI - PMC - PubMed
-
- NCT00296231. Nasal high frequency ventilation in preterm infants: a pilot study. clinicaltrials.gov/show/NCT00296231 (first received 24 February 2006). [CENTRAL: CN-01481122]
Cools 2009 {published data only}
-
- Cools F, Askie LM, Offringa M, the Prevention of Ventilator Induced Lung Injury collaborative study Group (PreVILIG Collaboration). Elective high-frequency oscillatory ventilation in preterm infants with respiratory distress syndrome: an individual patient data meta-analysis. BMC Pediatrics 2009;9:33. [DOI: ] [PMID: ] - PMC - PubMed
Czernik 2012 {published data only}
-
- Czernik C, Schmalisch G, Bührer C, Proquitté. Weaning of neonates from mechanical ventilation by use of nasopharyngeal high-frequency oscillatory ventilation: a preliminary study. Journal of Maternal-Fetal and Neonatal Medicine 2012;25(4):374-8. [DOI: 10.3109/14767058.2011.580401] [PMID: ] - DOI - PubMed
Gaertner 2021 {published data only}
-
- Gaertner VD, Waldmann AD, Davis PG, Bassler D, Springer L, Thomson J, et al. Transmission of oscillatory volumes into the preterm lung during noninvasive high-frequency ventilation. American Journal of Respiratory and Critical Care Medicine 2021;203(8):998-1005. [DOI: 10.1164/rccm.202007-2701OC] [PMID: ] - DOI - PubMed
He 2022 {published data only}
-
- He H, Wang M, Hao X, Chang X, Yan S. Effects of non-invasive high-frequency ventilation combined with inhaled NO on oxygenation function of premature infants with respiratory failure [无创高频通气联合吸入 NO 对呼吸衰竭早产儿氧合功能的影响]. Chongqing Medicine 2022;51(03):464-7. [DOI: 10.3969/j.issn.1671-8348.2022.03.022] - DOI
Hoehn 2000 {published data only}
Keel 2021 {published data only}
Klotz 2018 {published data only}
-
- DRKS00007171. Nasal noninvasive high frequency oscillatory ventilation in premature infants below 32 weeks gestational age - a pilot study [Nasal noninvasive high frequency oscillatory ventilation in premature infants below 32 weeks gestational age ‐ a pilot study ‐ nasal HFOV for premature infants]. trialsearch.who.int/Trial2.aspx?TrialID=DRKS00007171 (first received 16 June 2015). [CENTRAL: CN-01801701]
-
- Klotz D, Schneider H, Schumann S, Mayer B, Fuchs H. Non-invasive high-frequency oscillatory ventilation in preterm infants: a randomised controlled cross-over trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 2018;103(4):F1-5. [DOI: 10.1136/archdischild-2017-313190] [PMID: ] - DOI - PubMed
Kohlhauser 2005 {published data only}
Kugelman 2016 {published data only}
-
- Kugelman A. Minimal invasive respiratory support in neonatal intensive care units; 15th International Congress on Pediatric Pulmonary, 2016 June 23-26; Naples, Italy. Pediatric Pulmonology 2016;51(Suppl 43):S46-7. [DOI: 10.1002/ppul.23455] - DOI
Kugelman 2017 {published data only}
-
- Kugelman A. Controversies and update on non invasive ventilation in the NICU; 31st Annual North American Cystic Fibrosis Conference, 2017 November 2-4; Indianapolis, Indiana, USA. Pediatric Pulmonology 2017;52(Suppl 46):S73-4. [DOI: 10.1002/ppul.23837] - DOI
Lai 2022 {published data only}
Lin 2021 {published data only}
Liu 2021 {published data only}
-
- Liu YL, Shao MK, Wang ZS. The clinical value of nasal non-invasive high-frequency oscillatory ventilation with sequential heating and humidification high-flow nasal catheter ventilation in the treatment of very low birth weight RDS [经鼻无创高频振荡通气序贯加温湿化高流量鼻导管 通气治疗极低体重 RDS 的临床价值]. Inner Mongolia Medical Journal 2021;53(11):1300-2. [DOI: 10.16096/J.cnki.nmgyxzz.2021.53.11.007] [URL: caod.oriprobe.com/articles/62439237/The_Clinical_Value_of_Nasal_Non__inv...] - DOI
Loniewska 2019 {published data only}
Mukerji 2015 {published data only}
NCT01506401 {published data only}
-
- NCT01506401. The oscillation for acute respiratory distress syndrome (ARDS) treated early (OSCILLATE) trial. clinicaltrials.gov/study/NCT01506401 (first received 06 August 2015). [CENTRAL: CN-01534802]
NCT04327466 {published data only}
-
- NCT04327466. Effect of high frequency oscillatory highflow nasal cannula on desaturations and bradycardia in preterm infants [Effect of high frequency oscillatory highflow nasal cannula on desaturations and bradycardia in preterm infants: a randomized crossover trial]. clinicaltrials.gov/study/NCT04327466 (first received 12 March 2020). [CENTRAL: CN-02091196]
Renesme 2020 {published data only}
-
- NCT02030691. Tolerance of nHFPV versus nCPAP in neonatal respiratory distress [Tolerance of nasal high frequency percussive ventilation versus nasal CPAP in neonatal respiratory distress in term and preterm (> 33 weeks of gestation) neonates]. clinicaltrials.gov/show/NCT02030691 (first received 07 January 2014). [CENTRAL: CN-01480269]
-
- Renesme L, Dumas de la Roque E, Germain C, Chevrier A, Rebola M, Cramaregeas S, et al. Nasal high-frequency percussive ventilation vs nasal continuous positive airway pressure in newborn infants respiratory distress: a cross-over clinical trial. Pediatr Pulmonol 2020;55(10):2617-23. [DOI: 10.1002/ppul.24935] [PMID: ] - DOI - PubMed
Ruegger 2018 {published data only}
-
- ACTRN12616001516471. Nasal high-frequency oscillation to improve respiratory stability of preterm infants: a randomized crossover study. trialsearch.who.int/Trial2.aspx?TrialID=ACTRN12616001516471 (first received 03 November 2016). [CENTRAL: CN-01830516]
-
- Gaertner VD, Waldmann AD, Davis PG, Bassler D, Springer L, Thomson J, et al. Transmission of oscillatory volumes into the preterm lung during noninvasive high-frequency ventilation. American Journal of Respiratory and Critical care Medicine 2021;203(8):998-1005. [DOI: 10.1164/rccm.202007-2701OC] [PMID: ] - DOI - PubMed
-
- Ruegger CM, Lorenz L, Kamlin CO, Manley BJ, Owen LS, Bassler D, et al. The effect of noninvasive high-frequency oscillatory ventilation on desaturations and bradycardia in very preterm infants: a randomized crossover trial. Journal of Pediatrics 2018;201:269-73.e2. [DOI: 10.1016/J.JPEDS.2018.05.029] [PMID: ] - DOI - PubMed
Shi 2020 {published data only}
Teng 2022 {published data only}
-
- Teng Q, Liu Z, He X. Efficacy analysis of non-invasive high frequency oscillatory ventilation with ventilator in 42 cases of very low birth weight infants with respiratory distress syndrome [呼吸机无创高频振荡通气在极低出生体质量儿呼吸窘迫综合征42例中的疗效分析]. Anhui Medical and Pharmaceutical Journal 2022;26(1):172-5. [DOI: 10.3969/j.issn.1009-6469.2022.01.040] [URL: caod.oriprobe.com/articles/62515013/Efficacy_analysis_of_non_invasive_hi...] - DOI
Thatrimontrichai 2020 {published data only}
-
- Thatrimontrichai A, Sirianansopa K, Janjindamai W, Dissaneevate S, Maneenil G. Comparison of endotracheal reintubation between nasal high-frequency oscillation and continuous positive airway pressure in neonates. American Journal of Perinatology 2020;37(4):409-14. [DOI: 10.1055/s-0039-1679932] [PMID: ] - DOI - PubMed
Van der Hoeven 1998 {published data only}
Wang 2017 {published data only}
Wu 2021 {published data only}
-
- Wu HL, Lei YQ, Xie WP, Chen Q, Zheng YR. Nasal high-frequency oscillatory ventilation vs. nasal continuous positive airway pressure as therapy for postextubation respiratory failure in infants after congenital heart surgery. Frontiers in Pediatrics 2021;9:700632. [DOI: 10.3389/fped.2021.700632] [PMID: ] - DOI - PMC - PubMed
Yang 2021b {published data only}
-
- Yang L, Ming Y, Peng Z, Duan W. Early application of non-invasive high frequency oscillatory ventilation in very low birth weight infants with respiratory distress syndrome [无创高频振荡通气在极低出生体质量儿呼吸窘迫综合征的早期应用]. Chinese Journal of Applied Clinical Pediatrics 2021;36(20):1555-8. [DOI: 10.3760/cma.j.cn101070-20200602-00930] [WPRIM Database: wpr-908009] - DOI
Zhang 2020 {published data only}
-
- Zhang WX. Efficacy non-invasive high frequency oscillatory ventilation combined with early budesonide nebulization rehabilitation to prevent BPD in premature infants [无创高频震荡通气联合早期布地奈德雾化吸入 预防早产儿支气管肺发育不良的疗效]. Chinese Journal of Misdiagnosis 2020;15(10):445-8. [DOI: ]
Zheng 2020 {published data only}
-
- Zheng O, Yuan G, Zhang S, Zhou S. Clinical effects of transnasal non-invasive high frequency oscillating ventilation combined with caffeine in the treatment of apnea in very low birth weight infants [经鼻无创高频振荡通气联合咖啡因治疗极低出生体重儿 呼吸暂停的临床效果]. China and Foreign Medical Research 2020;18(22):18-20. [DOI: 10.14033/j.cnki.cfmr.2020.22.006] - DOI
References to ongoing studies
ChiCTR1900028092 {published data only}
-
- ChiCTR1900028092. Clinical study for multiple ventilation methods on graded respiratory support in neonates with respiratory distress syndrome. trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR1900028092 (first received 11 December 2019). [CENTRAL: CN-02435606]
ChiCTR2100045446 {published data only}
-
- ChiCTR2100045446. Application of high frequency oscillatory ventilation in premature infants [Noninvasive high-frequency oscillatory ventilation versus bi-level positive pressure ventilation in premature infants with respiratory failure: a randomized controlled study]. trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR2100045446 (first received 15 April 2021). [CENTRAL: CN-02349293]
CTRI/2021/10/037681 {published data only}
-
- CTRI/2021/10/037681. Comparison of non invasive ventilation in CPAP failure preterm infants with respiratory distress as a rescue mode [Non-invasive high-frequency oscillatory ventilation versus non-invasive intermittent mandatory ventilation as a rescue mode in preterm infants with respiratory distress on nasal CPAP - randomized trial]. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2021/10/037681 (first recived 29 October 2021). [DOI: ]
DRKS00005387 {published data only}
-
- DRKS00005387. Randomised crossover trial of different nasal support systems for the treatment of apnea of prematurity [Randomised crossover trial of different nasal support systems for the treatment of apnea of prematurity ‐ NASUS]. trialsearch.who.int/Trial2.aspx?TrialID=DRKS00005387 (first received 12 November 2013). [CENTRAL: CN-01814499]
DRKS00023438 {published data only}
-
- DRKS00023438. Influence of non-invasive positive pressure ventilation versus nasal high-frequency oscillation ventilation on parameters of oxygenation and ventilation in premature infants in the weaning phase after respiratory distress syndrome. trialsearch.who.int/Trial2.aspx?TrialID=DRKS00023438 (first received 11 May 2021). [CENTRAL: CN-02281427]
IRCT2016111930964N1 {published data only}
-
- IRCT2016111930964N1. Study of two non-invasive ventilation methods in the treatment of acute respiratory distress syndrome of infants [Efficacy of continuous positive oscillatory nasal airway pressure versus continuous positive nasal airway pressure in neonates with 28-34 weeks of gestational age with respiratory distress syndrome: a randomized controlled trial]. trialsearch.who.int/Trial2.aspx?TrialID=IRCT2016111930964N1 (first recived 30 December 2016). [CENTRAL: CN-01816554]
IRCT20180915041040N3 {published data only}
-
- IRCT20180915041040N3. Comparison of the effectiveness of nasal continuous positive airway pressure (NCPAP) therapy with combination of high-frequency oscillations and NCPAP in treatment of respiratory distress syndrome in infants. trialsearch.who.int/Trial2.aspx?TrialID=IRCT20180915041040N3 (first received 17 August 2019). [CENTRAL: CN-01972876]
IRCT20190416043290N2 {published data only}
-
- IRCT20190416043290N2. Comparison of two methods of respiratory support in neonates [Comparison of the consequences of using two methods of "continuous positive airway pressure" and "high frequency oscillation" in the treatment of respiratory distress in premature neonates]. trialsearch.who.int/Trial2.aspx?TrialID=IRCT20190416043290N2 (first received 17 Decmber 2020). [CENTRAL: CN-02239750]
IRCT20201222049795N1 {published data only}
-
- IRCT20201222049795N1. A comparison of the effect of nasal continuous positive airway pressure (NCPAP) vs nasal high-frequency oscillation (NHFO) in the treatment of respiratory distress in preterm infants. trialsearch.who.int/Trial2.aspx?TrialID=IRCT20201222049795N1 (first received 09 January 2021). [CENTRAL: CN-02241475]
IRCT20221120056556N1 {published data only}
-
- IRCT20221120056556N1. Comparison of the effect of nasal synchronized intermittent mandatory ventilation (NSIMV) with nasal high frequency oscillatory ventilation (NHFOV) in neonates requiring non-invasive mechanical ventilation in Mofid Pediatric Hospital. trialsearch.who.int/Trial2.aspx?TrialID=IRCT20221120056556N1 (first received 24 January 2023). [CENTRAL: CN-02521425]
NCT01277874 {published data only}
-
- NCT01277874. Oscillatory versus non-oscillatory nasal continuous airway pressure neonatal respiratory support. clinicaltrials.gov/study/NCT01277874 (first received 13 January 2011). [CENTRAL: CN-01502817]
NCT01852916 {published data only}
-
- NCT01852916. NHFOV versus NCPAP to prevent exubation failure [Nasal high frequency oscillatory ventilation (NHFOV) versus nasal continuous positive airway pressure (NCPAP) ventilation: a pilot trial]. clinicaltrials.gov/study/NCT01852916 (first received 17 April 2013). [CENTRAL: CN-01542143]
NCT02543125 {published data only}
-
- NCT02543125. Nasal high frequency oscillatory versus nasal intermittent positive pressure ventilation in neonate after extubation. clinicaltrials.gov/study/NCT02543125 (first received 21 August 2015). [CENTRAL: CN-01492062]
NCT03006354 {published data only}
-
- NCT03006354. nHFOV versus nCPAP in transient tachypnea of the newborn [Nasal high frequency oscillatory ventilation versus nasal continuous positive airway pressure in late‐preterm and term infants with transient tachypnea of the newborn: a randomized controlled trial]. clinicaltrials.gov/study/NCT03006354 (first received 28 December 2016). [CENTRAL: CN-01560940]
NCT03206489 {published data only}
-
- NCT03206489. Nasal high frequency oscillation for respiratory distress syndrome in preterm twins infants. clinicaltrials.gov/study/NCT03206489 (first recived 26 June 2017). [CN-01579637]
NCT03558737 {published data only}
-
- NCT03558737. Nasal high-frequency jet ventilation (nHFJV) following extubation in preterm infants. clinicaltrials.gov/study/NCT03558737 (first received 25 April 2018). [CENTRAL: CN-01660381]
NCT03711565 {published data only}
-
- NCT03711565. Oscillatory versus non-oscillatory nasal continuous airway pressure neonatal respiratory support. clinicaltrials.gov/study/NCT03711565 (first recived 15 October 2018). [CENTRAL: CN-01648720]
NCT03842462 {published data only}
-
- NCT03842462. NHFOV vs NIPPV vs nCPAP in preterm infants with respiratory distress syndrome. clinicaltrials.gov/study/NCT03842462 (first received 31 January 2019).
NCT04282369 {published data only}
-
- NCT04282369. Evaluation of the efficacy of four different non-invasive ventilation modes performed in the delivery room. clinicaltrials.gov/study/NCT04282369 (first received 17 February 2020).
NCT04323397 {published data only}
-
- NCT04323397. Nasal HFOV versus nasal SIPPV in neonate following extubation: RCT crossover study [Nasal high frequency oscillatory versus synchronized intermittent positive pressure ventilation in neonate following extubation: randomized controlled crossover study]. clinicaltrials.gov/study/NCT04323397 (first received 25 March 2020). [CENTRAL: CN-02091090]
NCT04905732 {published data only}
-
- NCT04905732. Nasal high-frequency oscillatory ventilation (NHFOV) for ventilated newborn infants with BPD [Nasal high‐frequency oscillatory ventilation (NHFOV) vs nasal continuous positive airway pressure (NCPAP) for ventilated newborn infants with BPD: a randomized controlled trial]. clinicaltrials.gov/study/NCT04905732 (first received 26 February 2021). [CENTRAL: CN-02277961]
NCT04914715 {published data only}
-
- NCT04914715. nHFOV versus invasive conventional ventilation for preterm neonates with respiratory distress syndrome [Effectiveness of non‐invasive high frequency oscillatory ventilation (nHFOV) versus invasive conventional ventilation for preterm neonates with respiratory distress syndrome]. clinicaltrials.gov/study/NCT04914715 (first received 17 March 2021). [CENTRAL: CN-02278163]
NCT05141435 {published data only}
-
- Li Y, Zhu X, Shi Y, for Noninvasive High-Frequency Oscillatory Ventilation Study Group. Non-invasive high-frequency oscillatory ventilation versus nasal continuous positive airway pressure in extremely preterm infants with respiratory distress syndrome: study protocol for a multi centre randomised controlled, superiority trial. BMJ Open 2023;13(e068450):1-6. [DOI: 10.1136/bmjopen-2022-068450] [PMID: ] - DOI - PMC - PubMed
-
- NCT05141435. NHFOV as primary support in very preterm infants with RDS [NHFOV vs nCPAP in very preterm infants with respiratory distress syndrome: a multi‐center, prospective, randomized, controlled clinical superior trial]. clinicaltrials.gov/study/NCT05141435 (first received 19 November 2021). [CENTRAL: CN-02345500]
NCT05493527 {published data only}
-
- NCT05493527. Noninvasive high frequency oscillatory ventilation as a post-extubation respiratory support in neonates [Noninvasive high frequency oscillatory ventilation as a post‐extubation respiratory support in preterm neonates: a randomized controlled trial]. clinicaltrials.gov/study/NCT05493527 (first received 03 August 2022). [CENTRAL: CN-02431730]
NCT05706428 {published data only}
-
- NCT05706428. Cardiorespiratory effects of nasal high frequency ventilation in neonates [Cardiorespiratory effects of nasal high frequency ventilation in moderate and late preterm infants with respiratory distress]. clinicaltrials.gov/study/NCT05706428 (first received 21 January 2023). [CENTRAL: CN-02519508]
Additional references
Abdel‐Latif 2011a
-
- Abdel-Latif ME, Osborn DA. Pharyngeal instillation of surfactant before the first breath for prevention of morbidity and mortality in preterm infants at risk of respiratory distress syndrome. Cochrane Database of Systematic Reviews 2011, Issue 3. Art. No: CD008311. [DOI: 10.1002/14651858.CD008311.pub2] - DOI - PubMed
Abdel‐Latif 2011b
-
- Abdel-Latif ME, Osborn DA. Laryngeal mask airway surfactant administration for prevention of morbidity and mortality in preterm infants with or at risk of respiratory distress syndrome. Cochrane Database of Systematic Reviews 2011, Issue 7. Art. No: CD008309. [DOI: 10.1002/14651858.CD008309.pub2] - DOI - PubMed
Abdel‐Latif 2012
Abdel‐Latif 2021
-
- Abdel-Latif ME, Davis PG, Wheeler KI, De Paoli AG, Dargaville PA. Surfactant therapy via thin catheter in preterm infants with or at risk of respiratory distress syndrome. Cochrane Database of Systematic Reviews 2021, Issue 5. Art. No: CD011672. [DOI: 10.1002/14651858.CD011672.pub2] - DOI - PMC - PubMed
Al Tawil 2011
-
- Al Tawil KI, Ahmed IA, Tawakol H, Saleem NB, Al Saif SA, Eldermerdash A. Management of pulmonary interstitial emphysema in a premature infant using nasopharyngeal high-frequency oscillatory ventilation. Journal of Pulmonary & Respiratory Medicine 2011;1(3):1-3. [DOI: 10.4172/2161-105X.1000108] - DOI
Allan 2010
Aly 2008
Ballard 1991
Bell 1978
Bhuta 1998
Brown 2011
Carlo 2008
Colaizy 2008
CONSORT Statement 2011
Cools 2009
-
- Cools F, Henderson-Smart DJ, Offringa M, Askie LM. Elective high frequency oscillatory ventilation versus conventional ventilation for acute pulmonary dysfunction in preterm infants. Cochrane Database of Systematic Reviews 2009, Issue 3. Art. No: CD000104. [DOI: 10.1002/14651858.CD000104.pub3] - DOI - PubMed
Cools 2010
-
- Cools F, Askie LM, Offringa M, Asselin JM, Calvert SA, Courtney SE, et al, PreVILIG collaboration. Elective high-frequency oscillatory versus conventional ventilation in preterm infants: a systematic review and meta-analysis of individual patients' data. Lancet 2010;375(9731):2082-91. [DOI: 10.1016/S0140-6736(10)60278-4] [PMID: ] - DOI - PubMed
Covidence 2023 [Computer program]
-
- Covidence systematic review software. Veritas Health Innovation, Version Accessed 8 April 2023. Melbourne, Australia: Veritas Health Innovation, 2023. Available at www.covidence.org.
Davis 2003
De Luca 2010
De Luca 2021
DiBlasi 2011
Donn 2009
EAPS 2020
-
- The European Academy of Paediatrics (EAP), The European Society of Paediatric and Neonatal Intensive Care (ESPNIC), The European Society for Paediatric Research (ESPR). In: The 8th Congress of the European Academy of Paediatric Societies - EAPS 2020. 2020. [DOI: 10.3389/978-2-88966-540-2] - DOI
EAPS 2021
-
- European Academy of Paediatrics (EAP). In: EAP 2021 Congress and Master Course. 2021. [DOI: 10.3389/978-2-88966-546-4] - DOI
EAPS 2022
-
- The European Academy of Paediatrics (EAP), The European Society for Paediatric Research (ESPR), The European Society of Paediatric and Neonatal Intensive Care (ESPNIC). In: 9th Congress of the European Academy of Paediatric Societies. 2022. [URL: eaps2022.kenes.com/wp-content/uploads/sites/135/2023/03/9782889710249-1.pdf]
Egger 1997
Fischer 2015
Ghazanshahi 1986
Gough 2012
GRADEpro GDT [Computer program]
-
- GRADEpro Guideline Development Tool [accessed 22 June 2023]. GRADE Working Group. Hamilton (ON): McMaster University and Evidence Prime.
Greenough 2002
Habre 2010
Haidar 2021
-
- Haidar Shehadeh AM. Non-invasive high flow oscillatory ventilation in comparison with nasal continuous positive pressure ventilation for respiratory distress syndrome, a literature review. Journal of Maternal-Fetal & Neonatal Medicine 2021;34(17):2900-9. [DOI: 10.1080/14767058.2019.1671332] [PMID: ] - DOI - PubMed
Higgins 2011
-
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). Cochrane, 2011. Available from training.cochrane.org/handbook/archives/v5.1.
Higgins 2023
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane, 2023. Available from www.training.cochrane.org/handbook.
Ho 2020a
Ho 2020b
Hodgson 2023
International Committee 2005
Jobe 2001
Keszler 1991
-
- Keszler M, Donn SM, Bucciarelli RL, Alverson DC, Hart M, Lunyong V, et al. Multicenter controlled trial comparing high-frequency jet ventilation and conventional mechanical ventilation in newborn infants with pulmonary interstitial emphysema. Journal of Pediatrics 1991;119(1 Pt 1):85-93. [DOI: 10.1016/s0022-3476(05)81046-7] [PMID: ] - DOI - PubMed
Keszler 1997
Lemyre 2023
-
- Lemyre B, Deguise M-O, Benson P, Kirpalani H, De Paoli AG, Davis PG. Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for preterm neonates after extubation. Cochrane Database of Systematic Reviews 2023, Issue 7. Art. No: CD003212. [DOI: 10.1002/14651858.CD003212.pub4] - DOI - PMC - PubMed
Li 2019
Li 2022
-
- Li J, Chen L, Shi Y. Nasal high-frequency oscillatory ventilation versus nasal continuous positive airway pressure as primary respiratory support strategies for respiratory distress syndrome in preterm infants: a systematic review and meta-analysis. European Journal of Pediatrics 2022;181(1):215-23. [DOI: 10.1007/s00431-021-04190-0] [PMID: ] - DOI - PubMed
Liberati 2009
-
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS Medicine 2009;6(6):e1000100. [DOI: 10.1136/bmj.b2700] [PMID: ] - DOI - PMC - PubMed
Moresco 2020
Mukerji 2013
Mukerji 2015
Mukerji 2016
Neumann 2014
Papile 1978
Pfister 2009
-
- Pfister RH, Soll R, Wiswell TE. Protein-containing synthetic surfactant versus protein-free synthetic surfactant for the prevention and treatment of respiratory distress syndrome. Cochrane Database of Systematic Reviews 2009, Issue 4. Art. No: CD006180. [DOI: 10.1002/14651858.CD006180.pub2] - DOI - PubMed
Ramanathan 2008
Reiterer 2013
-
- Reiterer F, Sellner A, Müller M, Rotky-Fast C, Maurer U, Resch B, et al. Neonatal and long-term morbidity of preterm infants (GA 24-28 weeks) with chronic lung disease up to the age of 6 years. In: Book of Abstracts of the XXXVII Alpe Adria Meeting of Perinatal Medicine 2013;34-34.-XXXV. 2013 September 20-21 . Slovenia, 2013.
Reuter 2014
RevMan 2024 [Computer program]
-
- Review Manager (RevMan Web). Version 7.2.0. The Cochrane Collaboration, 2024. Available at revman.cochrane.org.
Rojas‐Reyes 2012
Rojas‐Reyes 2015
Sarnaik 2011
-
- Sarnaik AP, Clark JA. Respiratory distress and failure. In: Nelson Textbook of Paediatrics . 19th edition. Philadelphia, PA: Elsevier/Saunders, 2011:314-33.e1.
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Shennan 1988
-
- Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics 1988;82(4):527-32. [PMID: ] - PubMed
Singer 1997
Singer 2001
-
- Singer LT, Siegel AC, Lewis B, Hawkins S, Yamashita T, Baley J. Preschool language outcomes of children with history of bronchopulmonary dysplasia and very low birth weight. Journal of Developmental and Behavioral Pediatrics 2001;22(1):19-26. [DOI: 10.1097/00004703-200102000-00003] [PMID: ] - DOI - PMC - PubMed
Soll 2010
Stedman 2000
-
- Stedman T. Stedman's Medical Dictionary. 27th edition. Baltimore, MD: Lippincott Williams & Wilkins, 2000.
Subramaniam 2021
Sweet 2017
Vohr 2000
-
- Vohr BR, Wright LL, Dusick AM, Mele L, Verter J, Steichen JJ, et al. Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Development Neonatal Research Network, 1993-1994. Pediatrics 2000;105(6):1216-26. [DOI: 10.1542/peds.105.6.1216] [PMID: ] - DOI - PubMed
Wheeler 2007
Yang 2018
-
- Yang Y-L, Wu B-Q, Su J-Z, Yang L, Zhong G-C, Liu L. Clinical efficacy of nasal high-frequency ventilation in treatment of neonatal respiratory distress syndrome: a meta-analysis. Zhongguo dang dai er ke za zhi [Chinese Journal of Contemporary Pediatrics] 2018;20(11):897-903. [DOI: 10.7499/j.issn.1008-8830.2018.11.004] [PMID: ] - DOI - PMC - PubMed
Yoder 2000
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

