Microglial TNFα controls daily changes in synaptic GABAARs and sleep slow waves
- PMID: 38695719
- PMCID: PMC11070559
- DOI: 10.1083/jcb.202401041
Microglial TNFα controls daily changes in synaptic GABAARs and sleep slow waves
Abstract
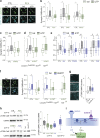
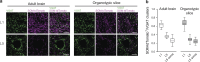
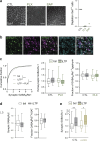
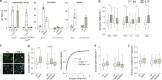
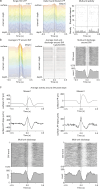
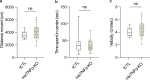
Microglia sense the changes in their environment. How microglia actively translate these changes into suitable cues to adapt brain physiology is unknown. We reveal an activity-dependent regulation of cortical inhibitory synapses by microglia, driven by purinergic signaling acting on P2RX7 and mediated by microglia-derived TNFα. We demonstrate that sleep induces microglia-dependent synaptic enrichment of GABAARs in a manner dependent on microglial TNFα and P2RX7. We further show that microglia-specific depletion of TNFα alters slow waves during NREM sleep and blunt memory consolidation in sleep-dependent learning tasks. Together, our results reveal that microglia orchestrate sleep-intrinsic plasticity of synaptic GABAARs, sculpt sleep slow waves, and support memory consolidation.
© 2024 Pinto et al.
Conflict of interest statement
Disclosures: The authors declare no competing financial interests.
Figures




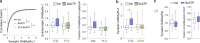



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

