Respiratory syncytial virus vaccination during pregnancy for improving infant outcomes
- PMID: 38695784
- PMCID: PMC11064886
- DOI: 10.1002/14651858.CD015134.pub2
Respiratory syncytial virus vaccination during pregnancy for improving infant outcomes
Abstract
Background: Respiratory syncytial virus (RSV) is a major cause of lower respiratory tract infections (LRTIs) in infants. Maternal RSV vaccination is a preventive strategy of great interest, as it could have a substantial impact on infant RSV disease burden. In recent years, the clinical development of maternal RSV vaccines has advanced rapidly.
Objectives: To assess the efficacy and safety of maternal respiratory syncytial virus (RSV) vaccination for preventing RSV disease in infants.
Search methods: We searched Cochrane Pregnancy and Childbirth's Trials Register and two other trials registries on 21 October 2022. We updated the search on 27 July 2023, when we searched MEDLINE, Embase, CENTRAL, CINAHL, and two trials registries. Additionally, we searched the reference lists of retrieved studies and conference proceedings. There were no language restrictions on our searches.
Selection criteria: We included randomised controlled trials (RCTs) comparing maternal RSV vaccination with placebo or no intervention in pregnant women of any age. The primary outcomes were hospitalisation with clinically confirmed or laboratory-confirmed RSV disease in infants. The secondary outcomes covered adverse pregnancy outcomes (intrauterine growth restriction, stillbirth, and maternal death) and adverse infant outcomes (preterm birth, congenital abnormalities, and infant death).
Data collection and analysis: We used standard Cochrane methods and assessed the certainty of evidence using the GRADE approach.
Main results: We included six RCTs (25 study reports) involving 17,991 pregnant women. The intervention was an RSV pre-F protein vaccine in four studies, and an RSV F protein nanoparticle vaccine in two studies. In all studies, the comparator was a placebo (saline, formulation buffer, or sterile water). We judged four studies at overall low risk of bias and two studies at overall high risk (mainly due to selection bias). All studies were funded by pharmaceutical companies. Maternal RSV vaccination compared with placebo reduces infant hospitalisation with laboratory-confirmed RSV disease (risk ratio (RR) 0.50, 95% confidence interval (CI) 0.31 to 0.82; 4 RCTs, 12,216 infants; high-certainty evidence). Based on an absolute risk with placebo of 22 hospitalisations per 1000 infants, our results represent 11 fewer hospitalisations per 1000 infants from vaccinated pregnant women (15 fewer to 4 fewer). No studies reported infant hospitalisation with clinically confirmed RSV disease. Maternal RSV vaccination compared with placebo has little or no effect on the risk of congenital abnormalities (RR 0.96, 95% CI 0.88 to 1.04; 140 per 1000 with placebo, 5 fewer per 1000 with RSV vaccination (17 fewer to 6 more); 4 RCTs, 12,304 infants; high-certainty evidence). Maternal RSV vaccination likely has little or no effect on the risk of intrauterine growth restriction (RR 1.32, 95% CI 0.75 to 2.33; 3 per 1000 with placebo, 1 more per 1000 with RSV vaccination (1 fewer to 4 more); 4 RCTs, 12,545 pregnant women; moderate-certainty evidence). Maternal RSV vaccination may have little or no effect on the risk of stillbirth (RR 0.81, 95% CI 0.38 to 1.72; 3 per 1000 with placebo, no difference with RSV vaccination (2 fewer to 3 more); 5 RCTs, 12,652 pregnant women). There may be a safety signal warranting further investigation related to preterm birth. This outcome may be more likely with maternal RSV vaccination, although the 95% CI includes no effect, and the evidence is very uncertain (RR 1.16, 95% CI 0.99 to 1.36; 6 RCTs, 17,560 infants; very low-certainty evidence). Based on an absolute risk of 51 preterm births per 1000 infants from pregnant women who received placebo, there may be 8 more per 1000 infants from pregnant women with RSV vaccination (1 fewer to 18 more). There was one maternal death in the RSV vaccination group and none in the placebo group. Our meta-analysis suggests that RSV vaccination compared with placebo may have little or no effect on the risk of maternal death (RR 3.00, 95% CI 0.12 to 73.50; 3 RCTs, 7977 pregnant women; low-certainty evidence). The effect of maternal RSV vaccination on the risk of infant death is very uncertain (RR 0.81, 95% CI 0.36 to 1.81; 6 RCTs, 17,589 infants; very low-certainty evidence).
Authors' conclusions: The findings of this review suggest that maternal RSV vaccination reduces laboratory-confirmed RSV hospitalisations in infants. There are no safety concerns about intrauterine growth restriction and congenital abnormalities. We must be careful in drawing conclusions about other safety outcomes owing to the low and very low certainty of the evidence. The evidence available to date suggests RSV vaccination may have little or no effect on stillbirth, maternal death, and infant death (although the evidence for infant death is very uncertain). However, there may be a safety signal warranting further investigation related to preterm birth. This is driven by data from one trial, which is not fully published yet. The evidence base would be much improved by more RCTs with substantial sample sizes and well-designed observational studies with long-term follow-up for assessment of safety outcomes. Future studies should aim to use standard outcome measures, collect data on concomitant vaccines, and stratify data by timing of vaccination, gestational age at birth, race, and geographical setting.
Copyright © 2024 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
EP has no conflicts of interest to declare. OB has no conflicts of interest to declare. FA has no conflicts of interest to declare. LB has regular interaction with pharmaceutical and other industrial partners. He has not received personal fees or other personal benefits. University Medical Center Utrecht (UMCU) has received major funding (> EUR 100,000 per industrial partner) for investigator‐initiated studies from AbbVie, MedImmune, AstraZeneca, Sanofi, Janssen, Pfizer, MSD, and MeMed Diagnostics. UMCU has received major funding for the RSV GOLD study from the Bill and Melinda Gates Foundation. UMCU has received major funding as part of the public private partnership IMI‐funded RESCEU and PROMISE projects with partners GSK, Novavax, Janssen, AstraZeneca, Pfizer, and Sanofi. UMCU has received major funding by Julius Clinical for participating in clinical studies sponsored by MedImmune and Pfizer. UMCU has received minor funding (EUR 1000 to 25,000 per industrial partner) for consultation and invited lectures by AbbVie, MedImmune, Ablynx, Bavaria Nordic, mAbxience, GSK, Novavax, Pfizer, Moderna, AstraZeneca, MSD, Sanofi, Genzyme, and Janssen. LB is the founding chairman of the ReSViNET Foundation. NM has no conflicts of interest to declare. MS has not received personal fees or other personal benefits. University Medical Center Utrecht has received major funding (> EUR 100,000 per industrial partner) for investigator‐initiated studies from AstraZeneca, Janssen Global Services, and Pfizer laboratories. JGW has received no personal fees or other personal benefits. She has been an investigator for clinical trials sponsored by pharmaceutical companies including AstraZeneca, Merck, Pfizer, Sanofi, and Janssen. All funds have been paid to UMCU. JGW participated in advisory boards of Janssen and Sanofi and was an invited speaker at a Sanofi‐sponsored symposium with fees paid to UMCU. KWMP has not received personal fees or any other personal benefits. University Medical Center Utrecht has received major funding (> EUR 100,000 per industrial partner) for an investigator‐initiated study from Pfizer.
Figures
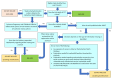
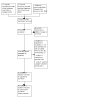
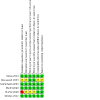







Update of
- doi: 10.1002/14651858.CD015134
References
References to studies included in this review
Bebia 2023 {published data only}
-
- Bebia Z, Reyes O, Jeanfreau R, Kantele A, De Leon RG, Garcia Sanchez M, et al. Safety and immunogenicity of an investigational respiratory syncytial virus vaccine (RSVPreF3) in mothers and their infants: a phase 2 randomized trial. Journal of infectious diseases 2023;228(3):299-310. [DOI: 10.1093/infdis/jiad024] [PMID: ] - DOI - PMC - PubMed
-
- EUCTR2019-001991-12-ES. Study of safety, reactogenicity and immunogenicity of GlaxoSmithKlinea€™s (GSK)Respiratory Syncytial Virus (RSV)maternal unadjuvanted vaccine in healthy pregnant women (aged 18 to 40 years) and their infants [A Phase II, randomised, observer-blind, placebo controlled multi-country study to assess the safety, reactogenicity and immunogenicity of a single intramuscular dose of GSK Biologicalsa€™ investigational RSV Maternal unadjuvanted vaccine (GSK3888550A), in healthy pregnant women aged 18 to 40 years and infants born to vaccinated mothers - RSV MAT-004]. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2019-001991-12-ES (first received 6 November 2019). [CENTRAL: CN-02169231]
-
- NCT04126213. Study of safety, reactogenicity and immunogenicity of glaxosmithkline's (GSK)respiratory syncytial virus (RSV)maternal unadjuvanted vaccine in healthy pregnant women (aged 18 to 40 years) and their infants [A phase II observer-blind study to assess safety, reactogenicity, and immunogenicity of GSK biologicals' investigational RSV maternal unadjuvanted vaccine (GSK3888550A), in healthy pregnant women and infants born to vaccinated mothers]. clinicaltrials.gov/show/nct04126213 (first received 15 October 2019). [CENTRAL: CN-01992840]
Dieussaert 2023 {published data only}
-
- Dieussaert I, Dormitzer PR, Kim JH, Luik S, Seidl C, Stegmann JU, et al. 126. Preterm birth signal in a maternal immunization study with a respiratory syncytial virus prefusion f protein vaccine candidate. In: Abstract Booklet RSVVW'23. 2023.
-
- EUCTR2020-001355-40-FI. A phase III double-blind study to assess safety and efficacy of an RSV Maternal unadjuvanted vaccine, in pregnant women and infants born to vaccinated mothers [A phase III, randomized, double-blind, placebo-controlled multi-country study to demonstrate efficacy of a single dose of unadjuvanted RSV Maternal vaccine, administered IM to pregnant women 18 to 49 years of age, for prevention of RSV associated LRTIs in their infants up to 6 months of age - RSV MAT-009]. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2020-001355-40-FI (first received 2 November 2020). [CENTRAL: CN-02255422]
-
- NCT04605159. A phase iii double-blind study to assess safety and efficacy of an rsv maternal unadjuvanted vaccine, in pregnant women and infants born to vaccinated mothers [A phase III, randomized, double-blind, placebo-controlled multi-country study to demonstrate efficacy of a single dose of unadjuvanted RSV maternal vaccine, administered IM to pregnant women 18 to 49 years of age, for prevention of RSV associated LRT is in their infants up to 6 months of age]. clinicaltrials.gov/show/NCT04605159 (first received 27 October 2020). [CENTRAL: CN-02196937]
-
- RSVPreF3 OA. Sponsor briefing document. Vaccines and related biological products advisory committee. Meeting Briefing Document - Sponsor GSK (fda.gov/media/165621/download) 2023.
Kampmann 2023 {published data only}
-
- EUCTR2019-002943-85-DK. A trial to evaluate the efficacy and safety of a respiratory syncytial virus (RSV) prefusion F subunit vaccine in infants born to women vaccinated during pregnancy [A phase 3, randomized, double-blinded, placebo-controlled trial to evaluate the efficacy and safety of a respiratory syncytial virus (RSV) prefusion F subunit vaccine in infants born to women vaccinated during pregnancy]. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2019-002943-85-DK (first received 4 March 2020). [CENTRAL: CN-02186793]
-
- NCT04424316. A trial to evaluate the efficacy and safety of RSVpreF in infants born to women vaccinated during pregnancy [A phase 3, randomized, double-blinded, placebo-controlled trial to evaluate the efficacy and safety of a respiratory syncytial virus (RSV) prefusion F subunit vaccine in infants born to women vaccinated during pregnancy]. clinicaltrials.gov/show/NCT04424316 (first received 9 June 2020). [CENTRAL: CN-02118732]
-
- PACTR202101800162440. A phase 3, randomized, double-blinded, placebo-controlled trial to evaluate the efficacy and safety of a respiratory syncytial virus (RSV) prefusion F subunit vaccine in infants born to women vaccinated during pregnancy. trialsearch.who.int/Trial2.aspx?TrialID=PACTR202101800162440 (first received 11 October 2020).
-
- Saleem S. Pfizer announces positive top-line data of phase 3 global maternal immunization trial for its bivalent respiratory syncytial virus (RSV) vaccine candidate. Neonatology Today 2022;17(11):131-4.
Madhi 2020 {published data only}
-
- CTRI/2017/09/009821. A study to determine the safety and effectiveness of the RSV f vaccine to protect infants via maternal vaccination [Randomized, observer-blind, placebo-controlled, group-sequential study to determine the immunogenicity and safety of a respiratory syncytial virus (RSV) F nanoparticle vaccine with aluminum in healthy third-trimester pregnant women; and safety and efficacy of maternally transferred antibodies in preventing RSV disease in their infants. - RSV-M-301]. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2017/09/009821 (first received 18 August 2017). [CENTRAL: CN-01892824]
-
- Fries L, Tomas DN, Smith G, Plested J, Piedra P, Patel N, et al. Progress toward a vaccine for maternal immunization to prevent respiratory syncytial virus (RSV) lower respiratory tract illness (LRTI) in infants. Open Forum Infectious Diseases 2018;5:S765-6. [CENTRAL: CN-01995193] [EMBASE: 629442701]
-
- Fries LF, Cho I, Thomas DN, Wen JL, Spindler MS, Fix AB, et al. Third trimester immunization with an respiratory syncytial virus F protein vaccine for the prevention of RSV lower respiratory tract infection in infants. Open Forum Infectious Diseases 2019;6:S921-2. [CENTRAL: CN-02073113] [EMBASE: 630692299]
-
- Lewnard JA, Fries LF, Cho I, Chen J, Laxminarayan R. Prevention of antimicrobial prescribing among infants following maternal vaccination against respiratory syncytial virus. Proceedings of the National Academy of Sciences of the United States of America 2022;119(12):e2112410119. [CENTRAL: CN-02386280] [EMBASE: 2017410282] [PMID: ] - PMC - PubMed
Muňoz 2019 {published data only}
-
- Muňoz FM, Swamy GK, Hickman SP, Agrawal S, Piedra PA, Glenn GM, et al. Safety and immunogenicity of a respiratory syncytial virus fusion (F) protein nanoparticle vaccine in healthy third-trimester pregnant women and their infants. Journal of Infectious Diseases 2019;220(11):1802-15. [CENTRAL: CN-02007966] [EMBASE: 629755883] [PMID: ] - PubMed
-
- NCT02247726. RSV F vaccine maternal immunization study in healthy third-trimester pregnant women [A phase ii randomized, observer-blind, placebo-controlled, study to evaluate the safety and immunogenicity of a respiratory syncytial virus (RSV) F nanoparticle vaccine with aluminum, in healthy third-trimester pregnant women and to assess the impact of maternal immunization on infant safety through one year of life]. clinicaltrials.gov/show/nct02247726 (first received 16 September 2014). [CENTRAL: CN-01583692]
Simões 2022 {published and unpublished data}
-
- NCT04032093. A phase 2b placebo-controlled, randomized study of a respiratory syncytial virus (RSV) vaccine in pregnant women [A phase 2b, randomized, placebo-controlled, observer-blinded trial to evaluate the safety, tolerability, and immunogenicity of a respiratory syncytial virus (RSV) vaccine in pregnant women 18 through 49 years of age and their infants]. clinicaltrials.gov/show/nct04032093 (first received 25 July 2019). [CENTRAL: CN-01965791]
-
- Simoes EA, Center KJ, Tita AT, Swanson KA, Radley D, Houghton J, et al. Prefusion F protein-based respiratory syncytial virus immunization in pregnancy. New England Journal of Medicine 2022;386(17):1615-26. [CENTRAL: CN-02396403] [EMBASE: 637892323] [PMID: ] - PubMed
-
- Simoes EA, Madhi SA, Center KJ, et al. 91. Establishing proof of concept for a bivalent RSVpreF subunit vaccine for maternal immunization. Open Forum Infectious Diseases 2022;9. [DOI: 10.1093/ofid/ofac492.016] - DOI
References to studies excluded from this review
Munoz 2003 {published data only}
-
- Muňoz FM, Piedra PA, Schoonover SL, Jewel AM, Glezen P. Maternal immunization with respiratory syncytial virus (RSV) vaccine - effect on breast milk antibodies [abstract]. In: Pediatric Academic Societies Annual Meeting; 2003 May 3-6; Seattle, Washington, USA. 2003. [CENTRAL: CN-00582761]
NCT03191383 {published data only}
-
- EUCTR2016-002733-30-ES. A study to evaluate the safety, reactogenicity and immunogenicity of the GSK investigational vaccine GSK3003891A in healthy pregnant women and infants born to vaccinated mothers [A Phase I/II, randomised, observer-blind, controlled multi-country study to assess the safety, reactogenicity and immunogenicity of a single intramuscular dose of GSK Biologicals’ investigational RSV vaccine (GSK3003891A), in healthy pregnant women aged 18 to 40 years and infants born to vaccinated mothers - RSV F-004]. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2016-002733-30-ES (first received 8 February 2017). [CENTRAL: CN-01879228]
-
- NCT03191383. A study to evaluate the safety, reactogenicity and immunogenicity of the glaxosmithkline (GSK) biologicals' respiratory syncytial virus (RSV) investigational vaccine (GSK3003891A) in healthy pregnant women and infants born to vaccinated mothers [An observer-blind study to assess the safety, reactogenicity and immunogenicity of GSK biologicals' investigational RSV vaccine (GSK3003891A), in healthy pregnant women and infants born to vaccinated mothers]. clinicaltrials.gov/show/nct03191383 (first received 15 June 2017). [CENTRAL: CN-01588706]
Wesley 2021 {published data only}
-
- Wesley C, Winckworth LC. Respiratory syncytial virus vaccination in pregnancy is not effective enough at reducing infant infections. Archives of Disease in Childhood: Education and Practice Edition 2022;107(5):389. [CENTRAL: CN-02260096] [EMBASE: 634425335] [PMID: ] - PubMed
References to studies awaiting assessment
Muňoz 2003b {published data only}
-
- Muňoz FM, Piedra PA, Glezen WP. Safety and immunogenicity of respiratory syncytial virus purified fusion protein-2 vaccine in pregnant women [Safety and immunogenicity of respiratory syncytial virus purified fusion protein-2 vaccine in pregnant women]. Vaccine 2003;21(24):3465-7. [CENTRAL: CN-00439159] [PMID: ] - PubMed
References to ongoing studies
NCT04980391 {published data only}
-
- CTRI/2021/10/037383. A study on the safety and immune response to an unadjuvanted RSV Maternal vaccine, in high risk pregnant women aged 15 to 49 years and infants born to the vaccinated mothers [A Phase III, double-blind, randomized, placebocontrolled study to evaluate the safety, reactogenicity and immune response of a single intramuscular dose of unadjuvanted RSV Maternal vaccine, in high risk pregnant women aged 15 to 49 years and infants born to the vaccinated mothers]. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2021/10/037383 (first received 2021). [CENTRAL: CN-02350152]
-
- EUCTR2021-000994-96-ES. A study on the safety and immune response to an unadjuvanted RSV Maternal vaccine, in high risk pregnant women aged 15 to 49 years and infants born to the vaccinated mothers [A Phase III, double-blind, randomized, placebo-controlled study to evaluate the safety, reactogenicity and immune response of a single intramuscular dose of unadjuvanted RSV Maternal vaccine, in high risk pregnant women aged 15 to 49 years and infants born to the vaccinated mothers. - RSV MAT-012]. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2021-000994-96-ES (first received 2021). [CENTRAL: CN-02329236]
-
- NCT04980391. A study on the safety and immune response to an unadjuvanted RSV maternal vaccine, in high risk pregnant women aged 15 to 49 years and infants born to the vaccinated mothers [A phase III, double-blind, randomized, placebo-controlled study to evaluate the safety, reactogenicity and immune response of a single intramuscular dose of unadjuvanted RSV maternal vaccine, in high risk pregnant women aged 15 to 49 years and infants born to the vaccinated mothers]. clinicaltrials.gov/show/NCT04980391 (first received 2021 Jul 28). [CENTRAL: CN-02291081]
Additional references
Abu Raya 2017
Carlisle 2017
-
- Carlisle JB. Data fabrication and other reasons for non-random sampling in 5087 randomised, controlled trial in anaesthetic and general medical journals. Anaesthesia 2017;72:944-52. - PubMed
CDC 2021
-
- Centers for Disease Control and Prevention Pregnancy and Vaccination. Vaccines during and after pregnancy. www.cdc.gov/vaccines/pregnancy/vacc-during-after.html (accessed prior to 15 October 2022).
CDC 2023
-
- Centers for Disease Control and Prevention. Transmission of RSV (Respiratory Syncytial Virus). www.cdc.gov/rsv/about/transmission.html (accessed prior to 7 July 2023).
Chu 2014a
Chu 2014b
Chu 2017a
Chu 2017b
-
- Chu HY, Tielsch J, Katz J, Magaret AS, Khatry S, LeClerq SC, et al. Transplacental transfer of maternal respiratory syncytial virus (RSV) antibody and protection against RSV disease in infants in rural Nepal. Journal of Clinical Virology 2017;95:90-5. [DOI: 10.1016/j.jcv.2017.08.017.] - DOI - PMC - PubMed
Faucette 2015
Glezen 1981
-
- Glezen WP, Paredes A, Allison JE, Taber LH, Frank AL. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. Journal of Pediatrics 1981;98:708-15. - PubMed
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed 2 September 2022. Hamilton (ON): McMaster University (developed by Evidence Prime), 2022. Available at gradepro.org.
GSK 2023
-
- Vaccines and Related Biological Products Advisory Committee. Meeting briefing document - sponsor GSK (fda.gov). www.fda.gov/media/165621/download 2023.
Gunatilaka 2021
Hall 2013
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2022
-
- Higgins JP, Eldridge S, Li T, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Available from training.cochrane.org/handbook/archive/v6.3 2022.
Li 2022
-
- Li Y, Wang X, Blau DM, Caballero MT, Feikin DR, Gill CJ, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet 2022;399(10340):2047-64. [DOI: 10.1016/S0140-6736(22)00478-0] - DOI - PMC - PubMed
Löwensteyn 2020
Madhi 2020
Malek 1996
-
- Malek A, Sager R, Kuhn P, Nicolaides KH, Schnei H. Evolution of maternofetal transport of immunoglobulins during human pregnancy. American Journal of Reproductive Immunology 1996;36:248-55. - PubMed
Mazur 2018
Mazur 2022
Muňoz 2019
-
- Muňoz FM, Swamy GK, Hickman SP, Agrawal S, Piedra PA, Glenn GM, et al. Safety and immunogenicity of a respiratory syncytial virus fusion (F) protein nanoparticle vaccine in healthy third-trimester pregnant women and their infants. Journal of Infectious Diseases 2019;220(11):1802-15. - PubMed
NCT04605159
-
- NCT04605159. A phase III double-blind study to assess safety and efficacy of an RSV maternal unadjuvanted vaccine, in pregnant women and infants born to vaccinated mothers (GRACE). clinicaltrials.gov/ct2/show/NCT04605159 (first received 27 October 2020).
Nyiro 2017
Ochola 2009
PATH snapshot
-
- PATH. PATH RSV vaccine and mAb snapshot - PATH vaccine resource library. www.path.org/resources/rsv-and-mab-trial-tracker (accessed 2 June 2022).
Pfizer 2023
-
- Respiratory Syncytial Virus Bivalent Stabilized Prefusion F Subunit Vaccine (RSVpreF) VRBPAC Briefing Document. www.fda.gov/media/168186/download (accessed 27 November 2023).
Phijffer 2022
-
- Phijffer E, Bruin O, Wildenbeest JG, Bont LJ, Sturkenboom MC, Van der Maas NA, et al. Respiratory syncytial virus vaccination during pregnancy for improving infant outcomes. Cochrane Database of Systematic Reviews 2022, Issue 10. Art. No: CD015134. [DOI: 10.1002/14651858.CD015134] - DOI - PMC - PubMed
Rau 2002
-
- Rau AT, Dhulia A, Wilson CG, Chopra GS, Sarkar PK. Transaplacentally transmitted anti-measles antibodies in term and preterm infants. Indian Pediatrics 2002;39(3):282-8. - PubMed
Review Manager 2020 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Scheltema 2018
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Stein 2017
van den Berg 2011
van den Berg 2014
Walsh 2018
WHO 2017
-
- World Health Organization. WHO preferred product characteristics for Respiratory Syncytial Virus (RSV) vaccines. World Health Organization. License: CC BY-NC-SA 3.0 IGO. apps.who.int/iris/handle/10665/258705 (accessed 17 October 2022).
WHO 2023
-
- World Health Organization. Fact sheet preterm birth. www.who.int/news-room/fact-sheets/detail/preterm-birth (accessed 27 November 2023).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

