A novel preclinical model of the normal human breast
- PMID: 38695983
- PMCID: PMC11065935
- DOI: 10.1007/s10911-024-09562-4
A novel preclinical model of the normal human breast
Abstract
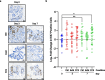
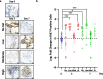
Improved screening and treatment have decreased breast cancer mortality, although incidence continues to rise. Women at increased risk of breast cancer can be offered risk reducing treatments, such as tamoxifen, but this has not been shown to reduce breast cancer mortality. New, more efficacious, risk-reducing agents are needed. The identification of novel candidates for prevention is hampered by a lack of good preclinical models. Current patient derived in vitro and in vivo models cannot fully recapitulate the complexities of the human tissue, lacking human extracellular matrix, stroma, and immune cells, all of which are known to influence therapy response. Here we describe a normal breast explant model utilising a tuneable hydrogel which maintains epithelial proliferation, hormone receptor expression, and residency of T cells and macrophages over 7 days. Unlike other organotypic tissue cultures which are often limited by hyper-proliferation, loss of hormone signalling, and short treatment windows (< 48h), our model shows that tissue remains viable over 7 days with none of these early changes. This offers a powerful and unique opportunity to model the normal breast and study changes in response to various risk factors, such as breast density and hormone exposure. Further validation of the model, using samples from patients undergoing preventive therapies, will hopefully confirm this to be a valuable tool, allowing us to test novel agents for breast cancer risk reduction preclinically.
Keywords: Explants; In vitro modelling; Normal breast; Prevention; Risk-reduction.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- CRUK. Breast Cancer Statistics. 2021; Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics/s.... Accessed Mar 2024.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

