Risk Stratification of Patients with Psoriatic Arthritis and Ankylosing Spondylitis for Treatment with Tofacitinib: A Review of Current Clinical Data
- PMID: 38696034
- PMCID: PMC11111604
- DOI: 10.1007/s40744-024-00662-5
Risk Stratification of Patients with Psoriatic Arthritis and Ankylosing Spondylitis for Treatment with Tofacitinib: A Review of Current Clinical Data
Abstract
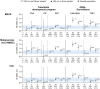
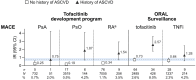
In this commentary, we review clinical data which helps inform individualized benefit-risk assessment for tofacitinib in patients with psoriatic arthritis (PsA) and ankylosing spondylitis (AS). ORAL Surveillance, a safety trial of patients ≥ 50 years of age with rheumatoid arthritis (RA) and cardiovascular risk factors, found increased rates of safety outcomes (including major adverse cardiovascular events [MACE], malignancies excluding non-melanoma skin cancer, and venous thromboembolism) with tofacitinib versus tumor necrosis factor inhibitors (TNFi). Post hoc analyses of ORAL Surveillance have identified subpopulations with different relative risk versus TNFi; higher risk with tofacitinib was confined to patients ≥ 65 years of age and/or long-time current/past smokers, and specifically for MACE, patients with a history of atherosclerotic cardiovascular disease (ASCVD). In patients without these risk factors, risk differences between tofacitinib and TNFi could not be detected. Given differences in demographics, pathophysiology, and comorbidities, we sought to examine whether the risk stratification observed in RA is also appropriate for PsA and AS. Data from the PsA tofacitinib development program show low absolute risk of safety outcomes in patients < 65 years of age and never smokers, and low MACE risk in patients with no history of ASCVD, consistent with results from ORAL Surveillance. No MACE, malignancies, or venous thromboembolism were reported in the tofacitinib AS development program. The mechanism of the ORAL Surveillance safety findings is unknown, and there are no similar prospective studies of sufficient size and duration. Accordingly, it is appropriate to use a precautionary approach and extrapolate differentiating risk factors identified from ORAL Surveillance (age ≥ 65 years, long-time current/past smoking, and history of ASCVD) to PsA and AS. We recommend an individualized approach to treatment decisions based on these readily identifiable risk factors, in line with updated labeling for Janus kinase inhibitors and international guidelines for the treatment of PsA and AS.Trial Registration: NCT02092467, NCT01262118, NCT01484561, NCT00147498, NCT00413660, NCT00550446, NCT00603512, NCT00687193, NCT01164579, NCT00976599, NCT01059864, NCT01359150, NCT02147587, NCT00960440, NCT00847613, NCT00814307, NCT00856544, NCT00853385, NCT01039688, NCT02281552, NCT02187055, NCT02831855, NCT00413699, NCT00661661, NCT01877668, NCT01882439, NCT01976364, NCT00678210, NCT01710046, NCT01241591, NCT01186744, NCT01276639, NCT01309737, NCT01163253, NCT01786668, NCT03502616.
Keywords: Age; Ankylosing spondylitis; Cardiovascular disease; Psoriatic arthritis; Smoking; Tofacitinib.
© 2024. The Author(s).
Conflict of interest statement
Lars Erik Kristensen has received grant/research support from Biogen, Janssen, Novartis, and UCB, and has received speaker’s fees and/or consultancy fees from AbbVie, Amgen, Biogen, Bristol Myers Squibb, Eli Lilly, Galapagos, Janssen, MSD, Novartis, Pfizer Inc, and UCB. Atul Deodhar has received grant/research support from AbbVie, Bristol Myers Squibb, Celgene, Eli Lilly, Janssen, Novartis, Pfizer Inc, and UCB; and consulting fees from AbbVie, Eli Lilly, Janssen, Novartis, Pfizer Inc, and UCB. Ying-Ying Leung has received speaker’s fees from and acted as a consultant for AbbVie, Eli Lilly, Janssen, Novartis, and Pfizer Inc. Ivana Vranic, Mahta Mortezavi, Lara Fallon, and Arne Yndestad are employees and shareholders of Pfizer Inc. Cassandra D. Kinch was an employee and shareholder of Pfizer Inc at the time of this work. Dafna D. Gladman has acted as a consultant for AbbVie, Amgen, Bristol Myers Squibb, Celgene, Eli Lilly, Galapagos, Gilead Sciences, Janssen, Novartis, Pfizer Inc, and UCB, and has received grants from AbbVie, Amgen, Celgene, Eli Lilly, Janssen, Novartis, Pfizer Inc, and UCB.
Figures
References
-
- Han C, Robinson DW, Jr, Hackett MV, Paramore LC, Fraeman KH, Bala MV. Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol. 2006;33:2167–2172. - PubMed
-
- Landgren AJ, Dehlin M, Jacobsson L, Bergsten U, Klingberg E. Cardiovascular risk factors in gout, psoriatic arthritis, rheumatoid arthritis and ankylosing spondylitis: a cross-sectional survey of patients in Western Sweden. RMD Open. 2021;7:e001568. doi: 10.1136/rmdopen-2021-001568. - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

