Treatment Patterns and Attrition With Lines of Therapy for Advanced Urothelial Carcinoma in the US
- PMID: 38696168
- PMCID: PMC11066705
- DOI: 10.1001/jamanetworkopen.2024.9417
Treatment Patterns and Attrition With Lines of Therapy for Advanced Urothelial Carcinoma in the US
Abstract
Importance: The treatment paradigm for advanced urothelial carcinoma (aUC) has undergone substantial transformation due to the introduction of effective, novel therapeutic agents. However, outcomes remain poor, and little is known about current treatment approaches and attrition rates for patients with aUC.
Objectives: To delineate evolving treatment patterns and attrition rates in patients with aUC using a US-based patient-level sample.
Design, setting, and participants: This retrospective cohort study used patient-level data from the nationwide deidentified electronic health record database Flatiron Health, originating from approximately 280 oncology clinics across the US. Patients included in the analysis received treatment for metastatic or local aUC at a participating site from January 1, 2011, to January 31, 2023. Patients receiving treatment for 2 or more different types of cancer or participating in clinical trials were excluded from the analysis.
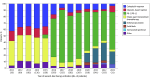
Main outcomes and measures: Frequencies and percentages were used to summarize the (1) treatment received in each line (cisplatin-based regimens, carboplatin-based regimens, programmed cell death 1 and/or programmed cell death ligand 1 [PD-1/PD-L1] inhibitors, single-agent nonplatinum chemotherapy, enfortumab vedotin, erdafitinib, sacituzumab govitecan, or others) and (2) attrition of patients with each line of therapy, defined as the percentage of patients not progressing to the next line.
Results: Of the 12 157 patients within the dataset, 7260 met the eligibility criteria and were included in the analysis (5364 [73.9%] men; median age at the start of first-line treatment, 73 [IQR, 66-80] years). All patients commenced first-line treatment; of these, only 2714 (37.4%) progressed to receive second-line treatment, and 857 (11.8%) advanced to third-line treatment. The primary regimens used as first-line treatment contained carboplatin (2241 [30.9%]), followed by PD-1/PD-L1 inhibitors (2174 [29.9%]). The PD-1/PD-L1 inhibitors emerged as the predominant choice in the second- and third-line (1412 of 2714 [52.0%] and 258 of 857 [30.1%], respectively) treatments. From 2019 onward, novel therapeutic agents were increasingly used in second- and third-line treatments, including enfortumab vedotin (219 of 2714 [8.1%] and 159 of 857 [18.6%], respectively), erdafitinib (39 of 2714 [1.4%] and 28 of 857 [3.3%], respectively), and sacituzumab govitecan (14 of 2714 [0.5%] and 34 of 857 [4.0%], respectively).
Conclusions and relevance: The findings of this cohort study suggest that approximately two-thirds of patients with aUC did not receive second-line treatment. Most first-line treatments do not include cisplatin-based regimens and instead incorporate carboplatin- or PD-1/PD-L1 inhibitor-based therapies. These data warrant the provision of more effective and tolerable first-line treatments for patients with aUC.
Conflict of interest statement
Figures



Comment in
References
-
- National Institute of Health, National Cancer Institute, Surveillance, Epidemiology, and End Results Program. Cancer stat facts: bladder cancer. 2023. Accessed October 10, 2023. https://seer.cancer.gov/statfacts/html/urinb.html
-
- Moore MJ, Iscoe N, Tannock IF. A phase II study of methotrexate, vinblastine, doxorubicin and cisplatin plus recombinant human granulocyte-macrophage colony stimulating factors in patients with advanced transitional cell carcinoma. J Urol. 1993;150(4):1131-1134. doi:10.1016/S0022-5347(17)35706-3 - DOI - PubMed
-
- Sternberg CN, de Mulder P, Schornagel JH, et al. ; EORTC Genito-Urinary Cancer Group . Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur J Cancer. 2006;42(1):50-54. doi:10.1016/j.ejca.2005.08.032 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

