Efficacy of Dequalinium Chloride vs Metronidazole for the Treatment of Bacterial Vaginosis: A Randomized Clinical Trial
- PMID: 38696172
- PMCID: PMC11066704
- DOI: 10.1001/jamanetworkopen.2024.8661
Efficacy of Dequalinium Chloride vs Metronidazole for the Treatment of Bacterial Vaginosis: A Randomized Clinical Trial
Abstract
Importance: Bacterial vaginosis (BV) is a common cause of vaginal infection. First-line treatments of BV are metronidazole and clindamycin. Due to the increase in antibiotic resistance, effective nonantibiotic treatments for BV are needed.
Objective: To examine whether dequalinium chloride, a broad-spectrum antiseptic, is noninferior to oral metronidazole for the treatment of BV.
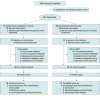
Design, setting, and participants: This phase 4, multicenter, triple-blind, double-dummy, parallel, noninferiority randomized clinical trial was conducted from July 29, 2021, to August 25, 2022, with a 1-month follow-up. Participants were premenopausal women 18 years or older with BV from 11 gynecologic practices and 1 hospital in Poland, Slovakia, and the Czech.
Intervention: Patients were randomized to treatment with dequalinium chloride vaginal tablets (10 mg once daily for 6 days) or oral metronidazole (500 mg twice daily for 7 days). Double-dummy medication kits contained vaginal and oral tablets with placebo and active medication.
Main outcomes and measures: The main outcome was the noninferiority margin (of 15 percentage points) in the absolute difference in clinical cure rates between dequalinium chloride and metronidazole 7 to 11 days after start of treatment (visit 1). Noninferiority was met if the lower 95% CI for the difference in clinical cure rate was less than 15 percentage points at visit 1.
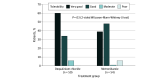
Results: A total of 147 women (mean [SD] age, 36.7 [9.0] years) were treated with dequalinium chloride (n = 72) or metronidazole (n = 75). The clinical cure rates at visit 1 were 64 of 69 (92.8%) for dequalinium chloride vs 69 of 74 (93.2%) for metronidazole in the intention-to-treat population, whereas in the per-protocol population, cure rates were 54 of 58 (93.1%) for dequalinium chloride vs 48 of 53 (90.6%) for metronidazole. The treatment differences of -0.5 percentage points (95% CI, -10.8 to 9.8 percentage points; P = .002) in the intention-to-treat population and 2.5 percentage points (95% CI, -9.4 to 14.4 percentage points; P = .001) in the per-protocol population confirmed the noninferiority of dequalinium chloride. The tolerability of dequalinium chloride was rated as very good by 30 of 50 patients (60.0%) but only by 21 of 54 (38.9%) for metronidazole. Three patients in the metronidazole group suspended treatment due to an adverse event.
Conclusions and relevance: This randomized clinical trial showed that dequalinium chloride was not inferior to metronidazole for the treatment of BV. Dequalinium chloride had a similarly high cure rate but with better tolerability and fewer adverse events. With a similar efficacy to metronidazole and clindamycin, dequalinium chloride warrants consideration as first-line treatment for BV to help reduce antibiotic consumption.
Trial registration: EudraCT: 2020-002489-15.
Conflict of interest statement
Figures

Comment in
-
Dequalinium Chloride-An Emerging Option in the Sparse Landscape of Bacterial Vaginosis Therapies.JAMA Netw Open. 2024 May 1;7(5):e248606. doi: 10.1001/jamanetworkopen.2024.8606. JAMA Netw Open. 2024. PMID: 38696175 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

