Smoking Cessation After Initial Treatment Failure With Varenicline or Nicotine Replacement: A Randomized Clinical Trial
- PMID: 38696203
- PMCID: PMC11066767
- DOI: 10.1001/jama.2024.4183
Smoking Cessation After Initial Treatment Failure With Varenicline or Nicotine Replacement: A Randomized Clinical Trial
Abstract
Importance: Most people who smoke do not quit on their initial attempt.
Objective: To determine the best subsequent strategy for nonabstinence following initial treatment with varenicline or combined nicotine replacement therapy (CNRT).
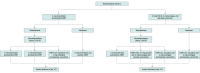
Design, setting, and participants: Using a double-blind, placebo-controlled, sequential multiple assignment randomized trial, 490 volunteers were randomized to receive 6 weeks of varenicline or CNRT. After 6 weeks, nonabstainers were rerandomized to continue, switch, or increase medication dosage for 6 additional weeks. The study was conducted from June 2015 through October 2019 in a Texas tobacco treatment clinic.
Interventions: The initial treatment was 2 mg/d of varenicline or the combined replacement therapy of a 21-mg patch plus 2-mg lozenge. The rerandomized participants either continued with their initial therapies, switched between varenicline and CNRT, or increased dosages either to 3-mg or more of varenicline or to a 42-mg patch and lozenges. All received weekly brief counseling.
Main outcomes and measures: Biochemically verified 7-day point prevalence abstinence at the end of treatment at 12 weeks.
Results: The 490 randomized participants (210 female [43%], 287 non-Hispanic White [58%], mean age, 48.1 years) smoked an average of 20 cigarettes per day. After the first phase, 54 participants in the CNRT group were abstinent and continued their therapy; of the 191 who were not abstinent, 151 were rerandomized, and the 40 who did not return for rerandomization were assigned to continue their initial CNRT condition in phase 2. The end-of-treatment abstinence rate for the 191 phase 1 nonabstainers was 8% (95% credible interval [CrI], 6% to 10%) for the 90 (47%) who continued at the dosage condition, 14% (CrI, 10% to 18%) for the 50 (33%) who increased their dosage, and 14% (95% CrI, 10% to 18%) for the 51 (34%) who switched to varenicline (absolute risk difference [RD], 6%; 95% CrI, 6% to 11%) with more than 99% posterior probability that either strategy conferred benefit over continuing the initial dosage. After the first phase, 88 participants in the varenicline group were abstinent and continued their therapy; of the 157 who were not abstinent, 122 were rerandomized and 35 who did not return for rerandomization were assigned to continue with the varenicline condition. The end-of-treatment abstinence rate for the 157 phase 1 nonabstainers was 20% (95% CrI, 16% to 26%) for the 39 (32%) who increased their varenicline dosage, 0 (95% CrI, 0 to 0) for the 41 (34%) who switched CNRT, and 3% (95% CrI, 1% to 4%) for the 77 (49%) who were assigned to the continued varenicline condition (absolute RD, -3%; 95% CrI, -4% to -1%) with more than 99% posterior probability that continuing varenicline at the initial dosage was worse than switching to a higher dosage. Furthermore, increasing the varenicline dosage had an absolute RD of 18% (95% CrI, 13% to 24%) and a more than 99% posterior probability of conferring benefit. The secondary outcome of continuous abstinence at 6 months indicated that only increased dosages of the CNRT and varenicline provided benefit over continuation of the initial treatment dosages.
Conclusions and relevance: For individuals who smoked but did not achieve abstinence after treatment with varenicline, increasing the dosage enhanced abstinence vs continuing, whereas for nonabstainers initially treated with CNRT, a dosage increase or switch to varenicline enhanced abstinence and may be viable rescue strategies.
Trial registration: ClinicalTrials.gov Identifier: NCT02271919.
Conflict of interest statement
Figures

Comment in
-
In persons smoking after 6 wk of varenicline or combined NRT, increasing dose or switching combined NRT to varenicline increased cessation.Ann Intern Med. 2024 Sep;177(9):JC106. doi: 10.7326/ANNALS-24-01842-JC. Epub 2024 Sep 3. Ann Intern Med. 2024. PMID: 39222501
References
-
- The Health Consequences of Smoking–50 Years of Progress: A Report of the Surgeon General. Centers for Disease Control and Prevention; 2014. Accessed January 2024. https://www.ncbi.nlm.nih.gov/books/NBK294315 - PubMed
-
- US Department of Health and Human Services . Smoking Cessation: A Report of the Surgeon General. 2020. Accessed January 2024. https://www.cdc.gov/tobacco/sgr/2020-smoking-cessation/index.html
-
- Fiore MC, Jaen CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update, clinical practice guideline. Updated March 4, 2015. Accessed June 13, 2022. https://wayback.archive-it.org/7993/20180125032208/https:/www.fda.gov/Sc...
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

