Measurable Residual FLT3 Internal Tandem Duplication Before Allogeneic Transplant for Acute Myeloid Leukemia
- PMID: 38696205
- PMCID: PMC11066770
- DOI: 10.1001/jamaoncol.2024.0985
Measurable Residual FLT3 Internal Tandem Duplication Before Allogeneic Transplant for Acute Myeloid Leukemia
Abstract
Importance: Persistence of FLT3 internal tandem duplication (ITD) in adults with acute myeloid leukemia (AML) in first complete remission (CR) prior to allogeneic hematopoietic cell transplant (HCT) is associated with increased relapse and death after transplant, but the association between the level of measurable residual disease (MRD) detected and clinical outcome is unknown.
Objective: To examine the association between pre-allogeneic HCT MRD level with relapse and death posttransplant in adults with AML in first CR.
Design, setting, and participants: In this cohort study, DNA sequencing was performed on first CR blood from patients with FLT3-ITD AML transplanted from March 2013 to February 2019. Clinical follow-up was through May 2022. Data were analyzed from October 2022 to December 2023.
Exposure: Centralized DNA sequencing for FLT3-ITD in pre-allogeneic HCT first CR blood using a commercially available kit.
Main outcomes and measures: The primary outcomes were overall survival and cumulative incidence of relapse, with non-relapse-associated mortality as a competing risk post-allogeneic HCT. Kaplan-Meier estimations (log-rank tests), Cox proportional hazards models, and Fine-Gray models were used to estimate the end points.
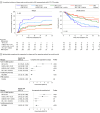
Results: Of 537 included patients with FLT3-ITD AML from the Pre-MEASURE study, 296 (55.1%) were female, and the median (IQR) age was 55.6 (42.9-64.1) years. Using the variant allele fraction (VAF) threshold of 0.01% or greater for MRD positivity, the results closely aligned with those previously reported. With no VAF threshold applied (VAF greater than 0%), 263 FLT3-ITD variants (median [range] VAF, 0.005% [0.0002%-44%]), and 177 patients (33.0%) with positive findings were identified. Multivariable analyses showed that residual FLT3-ITD was the variable most associated with relapse and overall survival, with a dose-dependent correlation. Patients receiving reduced-intensity conditioning without melphalan or nonmyeloablative conditioning had increased risk of relapse and death at any given level of MRD compared with those receiving reduced-intensity conditioning with melphalan or myeloablative conditioning.
Conclusions and relevance: This study provides generalizable and clinically applicable evidence that the detection of residual FLT3-ITD in the blood of adults in first CR from AML prior to allogeneic HCT is associated with an increased risk of relapse and death, particularly for those with a VAF of 0.01% or greater. While transplant conditioning intensification, an intervention not available to all, may help mitigate some of this risk, alternative approaches will be necessary for this high-risk population of patients who are underserved by the current standard of care.
Conflict of interest statement
Figures


Update of
-
Measurable Residual IDH1 before Allogeneic Transplant for Acute Myeloid Leukemia.medRxiv [Preprint]. 2023 Aug 1:2023.07.28.23293166. doi: 10.1101/2023.07.28.23293166. medRxiv. 2023. Update in: JAMA Oncol. 2024 Aug 1;10(8):1104-1110. doi: 10.1001/jamaoncol.2024.0985. PMID: 37577695 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

