BTK drives neutrophil activation for sterilizing antifungal immunity
- PMID: 38696257
- PMCID: PMC11178547
- DOI: 10.1172/JCI176142
BTK drives neutrophil activation for sterilizing antifungal immunity
Abstract
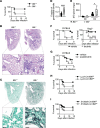
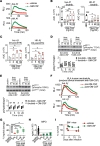
We describe a previously unappreciated role for Bruton's tyrosine kinase (BTK) in fungal immune surveillance against aspergillosis, an unforeseen complication of BTK inhibitors (BTKi) used for treating B cell lymphoid malignancies. We studied BTK-dependent fungal responses in neutrophils from diverse populations, including healthy donors, patients who were treated with BTKi, and X-linked agammaglobulinemia patients. Upon fungal exposure, BTK was activated in human neutrophils in a TLR2-, Dectin-1-, and FcγR-dependent manner, triggering the oxidative burst. BTK inhibition selectively impeded neutrophil-mediated damage to Aspergillus hyphae, primary granule release, and the fungus-induced oxidative burst by abrogating NADPH oxidase subunit p40phox and GTPase RAC2 activation. Moreover, neutrophil-specific Btk deletion in mice enhanced aspergillosis susceptibility by impairing neutrophil function, not recruitment or lifespan. Conversely, GM-CSF partially mitigated these deficits by enhancing p47phox activation. Our findings underline the crucial role of BTK signaling in neutrophils for antifungal immunity and provide a rationale for GM-CSF use to offset these deficits in patients who are susceptible.
Keywords: Fungal infections; Immunology; Infectious disease; Innate immunity; Neutrophils.
Figures





References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

