Angiogenic Gene Therapy for Refractory Angina: Results of the EXACT Phase 2 Trial
- PMID: 38696284
- PMCID: PMC11097950
- DOI: 10.1161/CIRCINTERVENTIONS.124.014054
Angiogenic Gene Therapy for Refractory Angina: Results of the EXACT Phase 2 Trial
Abstract
Background: XC001 is a novel adenoviral-5 vector designed to express multiple isoforms of VEGF (vascular endothelial growth factor) and more safely and potently induce angiogenesis. The EXACT trial (Epicardial Delivery of XC001 Gene Therapy for Refractory Angina Coronary Treatment) assessed the safety and preliminary efficacy of XC001 in patients with no option refractory angina.
Methods: In this single-arm, multicenter, open-label trial, 32 patients with no option refractory angina received a single treatment of XC001 (1×1011 viral particles) via transepicardial delivery.
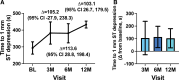
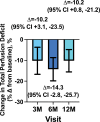
Results: There were no severe adverse events attributed to the study drug. Twenty expected severe adverse events in 13 patients were related to the surgical procedure. Total exercise duration increased from a mean±SD of 359.9±105.55 seconds at baseline to 448.2±168.45 (3 months), 449.2±175.9 (6 months), and 477.6±174.7 (12 months; +88.3 [95% CI, 37.1-139.5], +84.5 [95% CI, 34.1-134.9], and +115.5 [95% CI, 59.1-171.9]). Total myocardial perfusion deficit on positron emission tomography imaging decreased by 10.2% (95% CI, -3.1% to 23.5%), 14.3% (95% CI, 2.8%-25.7%), and 10.2% (95% CI, -0.8% to -21.2%). Angina frequency decreased from a mean±SD 12.2±12.5 episodes to 5.2±7.2 (3 months), 5.1±7.8 (6 months), and 2.7±4.8 (12 months), with an average decrease of 7.7 (95% CI, 4.1-11.3), 6.6 (95% CI, 3.5-9.7), and 8.8 (4.6-13.0) episodes at 3, 6, and 12 months. Angina class improved in 81% of participants at 6 months.
Conclusions: XC001 administered via transepicardial delivery is safe and generally well tolerated. Exploratory improvements in total exercise duration, ischemic burden, and subjective measures support a biologic effect sustained to 12 months, warranting further investigation.
Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT04125732.
Keywords: angiogenesis; coronary artery disease; genetic therapy; positron emission tomography; vascular endothelial growth factor A.
Conflict of interest statement
Figures



References
-
- Davies A, Fox K, Galassi AR, Banai S, Yla-Herttuala S, Luscher TF. Management of refractory angina: an update. Eur Heart J. 2021;42:269–283. doi: 10.1093/eurheartj/ehaa820 - PubMed
-
- Gallone G, Baldetti L, Tzanis G, Gramegna M, Latib A, Colombo A, Henry TD, Giannini F. Refractory angina: from pathophysiology to new therapeutic nonpharmacological technologies. JACC Cardiovasc Interv. 2020;13:1–19. doi: 10.1016/j.jcin.2019.08.055 - PubMed
-
- Povsic TJ, Henry TD, Ohman EM. Therapeutic approaches for the no-option refractory angina patient. Circ Cardiovasc Interv. 2021;14:e009002. doi: 10.1161/CIRCINTERVENTIONS.120.009002 - PubMed
-
- Mannheimer C, Camici P, Chester MR, Collins A, DeJongste M, Eliasson T, Follath F, Hellemans I, Herlitz J, Lüscher T, et al. . The problem of chronic refractory angina; report from the ESC joint study group on the treatment of refractory angina. Eur Heart J. 2002;23:355–370. doi: 10.1053/euhj.2001.2706 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

