Poloxamer-188 as a wetting agent for microfluidic resistive pulse sensing measurements of extracellular vesicles
- PMID: 38696491
- PMCID: PMC11065227
- DOI: 10.1371/journal.pone.0295849
Poloxamer-188 as a wetting agent for microfluidic resistive pulse sensing measurements of extracellular vesicles
Abstract
Introduction: Microfluidic resistive pulse sensing (MRPS) can determine the concentration and size distribution of extracellular vesicles (EVs) by measuring the electrical resistance of single EVs passing through a pore. To ensure that the sample flows through the pore, the sample needs to contain a wetting agent, such as bovine serum albumin (BSA). BSA leaves EVs intact but occasionally results in unstable MRPS measurements. Here, we aim to find a new wetting agent by evaluating Poloxamer-188 and Tween-20.
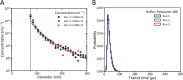
Methods: An EV test sample was prepared using an outdated erythrocyte blood bank concentrate. The EV test sample was diluted in Dulbecco's phosphate-buffered saline (DPBS) or DPBS containing 0.10% BSA (w/v), 0.050% Poloxamer-188 (v/v) or 1.00% Tween-20 (v/v). The effect of the wetting agents on the concentration and size distribution of EVs was determined by flow cytometry. To evaluate the precision of sample volume determination with MRPS, the interquartile range (IQR) of the particles transit time through the pore was examined. To validate that DPBS containing Poloxamer-188 yields reliable MRPS measurements, the repeatability of MRPS in measuring blood plasma samples was examined.
Results: Flow cytometry results show that the size distribution of EVs in Tween 20, in contrast to Poloxamer-188, differs from the control measurements (DPBS and DPBS containing BSA). MRPS results show that Poloxamer-188 improves the precision of sample volume determination compared to BSA and Tween-20, because the IQR of the transit time of EVs in the test sample is 11 μs, which is lower than 56 μs for BSA and 16 μs for Tween-20. Furthermore, the IQR of the transit time of particles in blood samples with Poloxamer-188 are 14, 16, and 14 μs, which confirms the reliability of MRPS measurements.
Conclusion: The solution of 0.050% Poloxamer-188 in DPBS does not lyse EVs and results in repeatable and unimpeded MRPS measurements.
Copyright: © 2024 Shahsavari et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors report no conflicts of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

