Major Group-B Enterovirus populations deleted in the noncoding 5' region of genomic RNA modulate activation of the type I interferon pathway in cardiomyocytes and induce myocarditis
- PMID: 38696536
- PMCID: PMC11093299
- DOI: 10.1371/journal.ppat.1012125
Major Group-B Enterovirus populations deleted in the noncoding 5' region of genomic RNA modulate activation of the type I interferon pathway in cardiomyocytes and induce myocarditis
Abstract
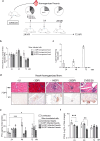
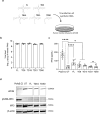
Major 5'-terminally deleted (5'TD) RNA forms of group-B coxsackievirus (CVB-5'TD) has been associated with myocarditis in both mice and humans. Although it is known that interferon-β (IFN-β) signaling is critical for an efficient innate immune response against CVB-induced myocarditis, the link between CVB-5'TD RNA forms and type I IFN signaling in cardiomyocytes remains to be explored. In a mouse model of CVB3/28-induced myocarditis, major early-emerging forms of CVB-5'TD RNA have been characterized as replicative viral populations that impair IFN-β production in the heart. Synthetic CVB3/28 RNA forms mimicking each of these major 5'TD virus populations were transfected in mice and have been shown to modulate innate immune responses in the heart and to induce myocarditis in mice. Remarkably, transfection of synthetic viral RNA with deletions in the secondary structures of the 5'-terminal CVB3 RNA domain I, modifying stem-loops "b", "c" or "d", were found to impair IFN-β production in human cardiomyocytes. In addition, the activation of innate immune response by Poly(I:C), was found to restore IFN-β production and to reduce the burden of CVB-5'TD RNA-forms in cardiac tissues, thereby reducing the mortality rate of infected mice. Overall, our results indicate that major early-emerging CVB3 populations deleted in the domain I of genomic RNA, in the 5' noncoding region, modulate the activation of the type I IFN pathway in cardiomyocytes and induce myocarditis in mice. These findings shed new light on the role of replicative CVB-5'TD RNA forms as key pathophysiological factors in CVB-induced human myocarditis.
Copyright: © 2024 Callon et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Major 5'terminally deleted enterovirus populations modulate type I IFN response in acute myocarditis patients and in human cultured cardiomyocytes.Sci Rep. 2020 Jul 20;10(1):11947. doi: 10.1038/s41598-020-67648-5. Sci Rep. 2020. PMID: 32686697 Free PMC article.
-
MicroRNA-30a Modulates Type I Interferon Responses to Facilitate Coxsackievirus B3 Replication Via Targeting Tripartite Motif Protein 25.Front Immunol. 2021 Jan 14;11:603437. doi: 10.3389/fimmu.2020.603437. eCollection 2020. Front Immunol. 2021. PMID: 33519812 Free PMC article.
-
Early Emergence of 5' Terminally Deleted Coxsackievirus-B3 RNA Forms Is Associated with Acute and Persistent Infections in Mouse Target Tissues.Vaccines (Basel). 2022 Jul 28;10(8):1203. doi: 10.3390/vaccines10081203. Vaccines (Basel). 2022. PMID: 36016091 Free PMC article.
-
The role of sex differences in autophagy in the heart during coxsackievirus B3-induced myocarditis.J Cardiovasc Transl Res. 2014 Mar;7(2):182-91. doi: 10.1007/s12265-013-9525-5. Epub 2013 Dec 10. J Cardiovasc Transl Res. 2014. PMID: 24323874 Free PMC article. Review.
-
Recombinant coxsackievirus vectors for prevention and therapy of virus-induced heart disease.Int J Med Microbiol. 2008 Jan;298(1-2):127-34. doi: 10.1016/j.ijmm.2007.08.010. Epub 2007 Sep 25. Int J Med Microbiol. 2008. PMID: 17897883 Review.
Cited by
-
An Exploration of the Relationship Between Gut Virome and Cardiovascular Disease: A Comprehensive Review.Rev Cardiovasc Med. 2025 Jun 24;26(6):36386. doi: 10.31083/RCM36386. eCollection 2025 Jun. Rev Cardiovasc Med. 2025. PMID: 40630433 Free PMC article. Review.
References
-
- Tracy S, Smithee S, Alhazmi A, Chapman N. Coxsackievirus can persist in murine pancreas by deletion of 5′ terminal genomic sequences: Coxsackievirus Persistence in the Pancreas. J Med Virol. 2015; 87(2):240–247. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

