Photoredox C(2)-Arylation of Indole- and Tryptophan-Containing Biomolecules
- PMID: 38696591
- PMCID: PMC11194849
- DOI: 10.1021/acs.orglett.4c01019
Photoredox C(2)-Arylation of Indole- and Tryptophan-Containing Biomolecules
Abstract
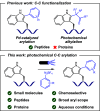
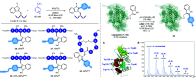
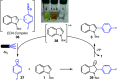
We introduce a novel and straightforward methodology for photoredox arylation of an indole scaffold using aryldiazonium salts under mild and metal-free conditions. Our approach enables the regioselective and chemoselective introduction of several aryl groups to the C(2) position of indoles and tryptophan, even in competition with other amino acids. This approach extends to the late-stage functionalization of peptides and lysozyme, heralding the unprecedented arylation of tryptophan residues in wild-type proteins and offering broad utility in chemical biology.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Bendi A.; Versha; Rajni; Singh L.; Taruna Insight into Indole-Based Heterocyclic Scaffolds: A Medicinal Chemistry Perspective. ChemistrySelect 2023, 8 (48), e20230387210.1002/slct.202303872. - DOI
-
- Bhattacharjee P.; Bora U. Organocatalytic Dimensions to the C-H Functionalization of the Carbocyclic Core in Indoles: A Review Update. Org. Chem. Front. 2021, 8 (10), 2343–2365. 10.1039/D0QO01466D. - DOI
-
- Ma J.; Feng R.; Dong Z. B. Recent Advances in Indole Synthesis and the Related Alkylation. Asian J. Org. Chem. 2023, 12 (6), e20230009210.1002/ajoc.202300092. - DOI
-
- Bradley S. A.; Lehka B. J.; Hansson F. G.; Adhikari K. B.; Rago D.; Rubaszka P.; Haidar A. K.; Chen L.; Hansen L. G.; Gudich O.; Giannakou K.; Lengger B.; Gill R. T.; Nakamura Y.; de Bernonville T. D.; Koudounas K.; Romero-Suarez D.; Ding L.; Qiao Y.; Frimurer T. M.; Petersen A. A.; Besseau S.; Kumar S.; Gautron N.; Melin C.; Marc J.; Jeanneau R.; O’Connor S. E.; Courdavault V.; Keasling J. D.; Zhang J.; Jensen M. K. Biosynthesis of Natural and Halogenated Plant Monoterpene Indole Alkaloids in Yeast. Nat. Chem. Biol. 2023, 19 (12), 1551–1560. 10.1038/s41589-023-01430-2. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

