Inverse Hypercorroles
- PMID: 38696617
- PMCID: PMC11094798
- DOI: 10.1021/acs.inorgchem.4c00344
Inverse Hypercorroles
Abstract
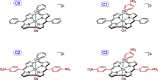
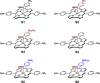
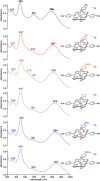
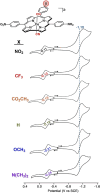
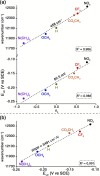
Ground-state and time-dependent density functional theory (TDDFT) calculations with the long-range-corrected, Coulomb-attenuating CAMY-B3LYP exchange-correlation functional and large, all-electron STO-TZ2P basis sets have been used to examine the potential "inverse hypercorrole" character of meso-p-nitrophenyl-appended dicyanidocobalt(III) corrole dianions. The effect is most dramatic for 5,15-bis(p-nitrophenyl) derivatives, where it manifests itself in intense NIR absorptions. The 10-aryl groups in these complexes play a modulatory role, as evinced by experimental UV-visible spectroscopic and electrochemical data for a series of 5,15-bis(p-nitrophenyl) dicyanidocobalt(III) corroles. TDDFT (CAMY-B3LYP) calculations ascribe these features clearly to a transition from the corrole's a2u-like HOMO (retaining the D4h irrep used for metalloporphyrins) to a nitrophenyl-based LUMO. The outward nature of this transition contrasts with the usual phenyl-to-macrocycle direction of charge transfer transitions in many hyperporphyrins and hypercorroles; thus, the complexes studied are aptly described as inverse hypercorroles.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Gouterman M. Spectra of porphyrins. J. Mol. Spectrosc. 1961, 6, 138–163. 10.1016/0022-2852(61)90236-3. - DOI
-
- Gouterman M.; Wagniére G. H.; Snyder L. C. Spectra of Porphyrins. Part II. Four-Orbital Model. J. Mol. Spectrosc. 1963, 11, 108–115. 10.1016/0022-2852(63)90011-0. - DOI
-
- Gouterman M.Optical Spectra and Electronic Structure of Porphyrins and Related Rings. In The Porphyrins; Dolphin D., Ed.; Academic Press: New York, 1978; Vol. III, Part A, pp. 1–165.
LinkOut - more resources
Full Text Sources
Miscellaneous

