Brain Metastasis from EGFR-Mutated Non-Small Cell Lung Cancer: Secretion of IL11 from Astrocytes Up-Regulates PDL1 and Promotes Immune Escape
- PMID: 38696655
- PMCID: PMC11234401
- DOI: 10.1002/advs.202306348
Brain Metastasis from EGFR-Mutated Non-Small Cell Lung Cancer: Secretion of IL11 from Astrocytes Up-Regulates PDL1 and Promotes Immune Escape
Abstract
Patients who have non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) mutations are more prone to brain metastasis (BM) and poor prognosis. Previous studies showed that the tumor microenvironment of BM in these patients is immunosuppressed, as indicated by reduced T-cell abundance and activity, although the mechanism of this immunosuppression requires further study. This study shows that reactive astrocytes play a critical role in promoting the immune escape of BM from EGFR-mutated NSCLC by increasing the apoptosis of CD8+ T lymphocytes. The increased secretion of interleukin 11(IL11) by astrocytes promotes the expression of PDL1 in BM, and this is responsible for the increased apoptosis of T lymphocytes. IL11 functions as a ligand of EGFR, and this binding activates EGFR and downstream signaling to increase the expression of PDL1, culminating in the immune escape of tumor cells. IL11 also promotes immune escape by binding to its intrinsic receptor (IL11Rα/glycoprotein 130 [gp130]). Additional in vivo studies show that the targeted inhibition of gp130 and EGFR suppresses the growth of BM and prolongs the survival time of mice. These results suggest a novel therapeutic strategy for treatment of NSCLC patients with EGFR mutations.
Keywords: astrocytes; brain metastasis; epidermal growth factor receptor; immune escape; interleukin‐11.
© 2024 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
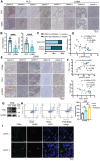


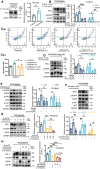

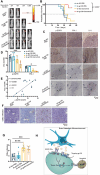
References
-
- Andrews D. W., Scott C. B., Sperduto P. W., Flanders A. E., Gaspar L. E., Schell M. C., Werner‐Wasik M., Demas W., Ryu J., Bahary J. P., Souhami L., Rotman M., Mehta M. P., Curran W. J. Jr., Lancet 2004, 363, 1665. - PubMed
-
- Shan Y., Eastwood M. P., Zhang X., Kim E. T., Arkhipov A., Dror R. O., Jumper J., Kuriyan J., Shaw D. E., Cell 2012, 149, 860. - PubMed
-
- Shin D. Y., Na I. I., Kim C. H., Park S., Baek H., Yang S. H., J Thorac Oncol 2014, 9, 195. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
