Distinct ultrastructural phenotypes of glial and neuronal alpha-synuclein inclusions in multiple system atrophy
- PMID: 38696728
- PMCID: PMC11531854
- DOI: 10.1093/brain/awae137
Distinct ultrastructural phenotypes of glial and neuronal alpha-synuclein inclusions in multiple system atrophy
Abstract
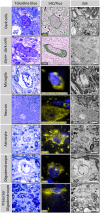
Multiple system atrophy is characterized pathologically by the accumulation of alpha-synuclein (aSyn) into glial cytoplasmic inclusions (GCIs). The mechanism underlying the formation of GCIs is not well understood. In this study, correlative light and electron microscopy was employed to investigate aSyn pathology in the substantia nigra and putamen of post-mortem multiple system atrophy brain donors. Three distinct types of aSyn immuno-positive inclusions were identified in oligodendrocytes, neurons and dark cells presumed to be dark microglia. Oligodendrocytes contained fibrillar GCIs that were consistently enriched with lysosomes and peroxisomes, supporting the involvement of the autophagy pathway in aSyn aggregation in multiple system atrophy. Neuronal cytoplasmic inclusions exhibited ultrastructural heterogeneity resembling both fibrillar and membranous inclusions, linking multiple systems atrophy and Parkinson's disease. The novel aSyn pathology identified in the dark cells, displayed GCI-like fibrils or non-GCI-like ultrastructures suggesting various stages of aSyn accumulation in these cells. The observation of GCI-like fibrils within dark cells suggests these cells may be an important contributor to the origin or spread of pathological aSyn in multiple system atrophy. Our results suggest a complex interplay between multiple cell types that may underlie the formation of aSyn pathology in multiple system atrophy brain and highlight the need for further investigation into cell-specific disease pathologies in multiple system atrophy.
Keywords: alpha-synuclein; correlative light and electron microscopy; disease pathology; multiple system atrophy; post-mortem human brain.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures




References
-
- Papp MI, Lantos PL. Accumulation of tubular structures in oligodendroglial and neuronal cells as the basic alteration in multiple system atrophy. J Neurol Sci. 1992;107:172–182. - PubMed
-
- Spillantini MG, Crowther RA, Jakes R, Cairns NJ, Lantos PL, Goedert M. Filamentous alpha-synuclein inclusions link multiple system atrophy with Parkinson’s disease and dementia with Lewy bodies. Neurosci Lett. 1998;251:205–208. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

