RNA aggregates harness the danger response for potent cancer immunotherapy
- PMID: 38697107
- PMCID: PMC11767857
- DOI: 10.1016/j.cell.2024.04.003
RNA aggregates harness the danger response for potent cancer immunotherapy
Abstract
Cancer immunotherapy remains limited by poor antigenicity and a regulatory tumor microenvironment (TME). Here, we create "onion-like" multi-lamellar RNA lipid particle aggregates (LPAs) to substantially enhance the payload packaging and immunogenicity of tumor mRNA antigens. Unlike current mRNA vaccine designs that rely on payload packaging into nanoparticle cores for Toll-like receptor engagement in immune cells, systemically administered RNA-LPAs activate RIG-I in stromal cells, eliciting massive cytokine/chemokine response and dendritic cell/lymphocyte trafficking that provokes cancer immunogenicity and mediates rejection of both early- and late-stage murine tumor models. In client-owned canines with terminal gliomas, RNA-LPAs improved survivorship and reprogrammed the TME, which became "hot" within days of a single infusion. In a first-in-human trial, RNA-LPAs elicited rapid cytokine/chemokine release, immune activation/trafficking, tissue-confirmed pseudoprogression, and glioma-specific immune responses in glioblastoma patients. These data support RNA-LPAs as a new technology that simultaneously reprograms the TME while eliciting rapid and enduring cancer immunotherapy.
Keywords: brain tumors; cancer immunotherapy; cancer vaccines; glioblastoma; lipid nanoparticles; mRNA; personalized therapy; translational therapeutics.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests D.A.M. holds ownership interest in iOncologi, Inc. E.J.S is a paid consultant for Siren Biotechnology. The manuscript discusses patented technologies from H.R.M.-G., P.C., S.Q., J.M., A.P.G., J.H., W.G.S., M.R., D.A.M., and E.J.S. Patented technologies are under option to license by iOncologi, Inc.
Figures




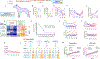

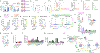
Update of
-
mRNA aggregates harness danger response for potent cancer immunotherapy.medRxiv [Preprint]. 2023 Mar 17:2023.03.12.23287108. doi: 10.1101/2023.03.12.23287108. medRxiv. 2023. Update in: Cell. 2024 May 9;187(10):2521-2535.e21. doi: 10.1016/j.cell.2024.04.003. PMID: 36993772 Free PMC article. Updated. Preprint.
Comment in
-
RNA delivery heats up cold tumours.Nat Rev Drug Discov. 2024 Jul;23(7):497. doi: 10.1038/d41573-024-00098-0. Nat Rev Drug Discov. 2024. PMID: 38858569 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

