The broken Alzheimer's disease genome
- PMID: 38697121
- PMCID: PMC11099344
- DOI: 10.1016/j.xgen.2024.100555
The broken Alzheimer's disease genome
Abstract
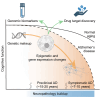
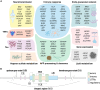
The complex pathobiology of late-onset Alzheimer's disease (AD) poses significant challenges to therapeutic and preventative interventions. Despite these difficulties, genomics and related disciplines are allowing fundamental mechanistic insights to emerge with clarity, particularly with the introduction of high-resolution sequencing technologies. After all, the disrupted processes at the interface between DNA and gene expression, which we call the broken AD genome, offer detailed quantitative evidence unrestrained by preconceived notions about the disease. In addition to highlighting biological pathways beyond the classical pathology hallmarks, these advances have revitalized drug discovery efforts and are driving improvements in clinical tools. We review genetic, epigenomic, and gene expression findings related to AD pathogenesis and explore how their integration enables a better understanding of the multicellular imbalances contributing to this heterogeneous condition. The frontiers opening on the back of these research milestones promise a future of AD care that is both more personalized and predictive.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

