A measles and rubella vaccine microneedle patch in The Gambia: a phase 1/2, double-blind, double-dummy, randomised, active-controlled, age de-escalation trial
- PMID: 38697170
- PMCID: PMC11099471
- DOI: 10.1016/S0140-6736(24)00532-4
A measles and rubella vaccine microneedle patch in The Gambia: a phase 1/2, double-blind, double-dummy, randomised, active-controlled, age de-escalation trial
Abstract
Background: Microneedle patches (MNPs) have been ranked as the highest global priority innovation for overcoming immunisation barriers in low-income and middle-income countries. This trial aimed to provide the first data on the tolerability, safety, and immunogenicity of a measles and rubella vaccine (MRV)-MNP in children.
Methods: This single-centre, phase 1/2, double-blind, double-dummy, randomised, active-controlled, age de-escalation trial was conducted in The Gambia. To be eligible, all participants had to be healthy according to prespecified criteria, aged 18-40 years for the adult cohort, 15-18 months for toddlers, or 9-10 months for infants, and to be available for visits throughout the follow-up period. The three age cohorts were randomly assigned in a 2:1 ratio (adults) or 1:1 ratio (toddlers and infants) to receive either an MRV-MNP (Micron Biomedical, Atlanta, GA, USA) and a placebo (0·9% sodium chloride) subcutaneous injection, or a placebo-MNP and an MRV subcutaneous injection (MRV-SC; Serum Institute of India, Pune, India). Unmasked staff ransomly assigned the participants using an online application, and they prepared visually identical preparations of the MRV-MNP or placebo-MNP and MRV-SC or placebo-SC, but were not involved in collecting endpoint data. Staff administering the study interventions, participants, parents, and study staff assessing trial endpoints were masked to treatment allocation. The safety population consists of all vaccinated participants, and analysis was conducted according to route of MRV administration, irrespective of subsequent protocol deviations. The immunogenicity population consisted of all vaccinated participants who had a baseline and day 42 visit result available, and who had no protocol deviations considered to substantially affect the immunogenicity endpoints. Solicited local and systemic adverse events were collected for 14 days following vaccination. Unsolicited adverse events were collected to day 180. Age de-escalation between cohorts was based on the review of the safety data to day 14 by an independent data monitoring committee. Serum neutralising antibodies to measles and rubella were measured at baseline, day 42, and day 180. Analysis was descriptive and included safety events, seroprotection and seroconversion rates, and geometric mean antibody concentrations. The trial was registered with the Pan African Clinical Trials Registry PACTR202008836432905, and is complete.
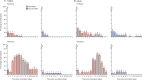
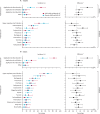
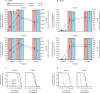
Findings: Recruitment took place between May 18, 2021, and May 27, 2022. 45 adults, 120 toddlers, and 120 infants were randomly allocated and vaccinated. There were no safety concerns in the first 14 days following vaccination in either adults or toddlers, and age de-escalation proceeded accordingly. In infants, 93% (52/56; 95% CI 83·0-97·2) seroconverted to measles and 100% (58/58; 93·8-100) seroconverted to rubella following MRV-MNP administration, while 90% (52/58; 79·2-95·2) and 100% (59/59; 93·9-100) seroconverted to measles and rubella respectively, following MRV-SC. Induration at the MRV-MNP application site was the most frequent local reaction occurring in 46 (77%) of 60 toddlers and 39 (65%) of 60 infants. Related unsolicited adverse events, most commonly discolouration at the application site, were reported in 35 (58%) of 60 toddlers and 57 (95%) of 60 infants that had received the MRV-MNP. All local reactions were mild. There were no related severe or serious adverse events.
Interpretation: The safety and immunogenicity data support the accelerated development of the MRV-MNP.
Funding: Bill & Melinda Gates Foundation.
Copyright © 2024 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests SH, DVM, MRP, and MR are employees of, or affiliated with, Micron Biomedical. All other authors declare no competing interests. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Figures

Comment in
-
Measles and rubella vaccine microneedle patch: new hope to reach the unreached children.Lancet. 2024 May 11;403(10439):1825-1827. doi: 10.1016/S0140-6736(24)00749-9. Epub 2024 Apr 29. Lancet. 2024. PMID: 38697172 No abstract available.
References
-
- WHO Measles and rubella strategic framework: 2021–2030. Feb 23, 2021. https://measlesrubellainitiative.org/measles-rubella-strategic-framework...
-
- WHO Measles vaccines: WHO position paper - April 2017. Wkly Epidemiol Rec. 2017;17:205–228.
-
- Winter AK, Moss WJ. Rubella. Lancet. 2022;399:1336–1346. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

