The fatty acid omega hydroxylase genes (CYP4 family) in the progression of metabolic dysfunction-associated steatotic liver disease (MASLD): An RNA sequence database analysis and review
- PMID: 38697309
- PMCID: PMC11774579
- DOI: 10.1016/j.bcp.2024.116241
The fatty acid omega hydroxylase genes (CYP4 family) in the progression of metabolic dysfunction-associated steatotic liver disease (MASLD): An RNA sequence database analysis and review
Abstract
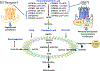
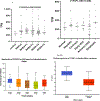
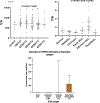
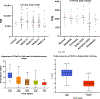
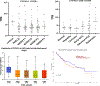
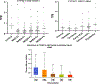
Fatty acid omega hydroxylase P450s consist of enzymes that hydroxylate various chain-length saturated and unsaturated fatty acids (FAs) and bioactive eicosanoid lipids. The human cytochrome P450 gene 4 family (CYP4) consists of 12 members that are associated with several human diseases. However, their role in the progression of metabolic dysfunction-associated fatty liver disease (MASLD) remains largely unknown. It has long been thought that the induction of CYP4 family P450 during fasting and starvation prevents FA-related lipotoxicity through FA metabolism to dicarboxylic acids that are chain-shortened in peroxisomes and then transported to the mitochondria for complete oxidation. Several studies have revealed that peroxisome succinate transported to the mitochondria is used for gluconeogenesis during fasting and starvation, and recent evidence suggests that peroxisome acetate can be utilized for lipogenesis and lipid droplet formation as well as epigenetic modification of gene transcription. In addition, omega hydroxylation of the bioactive eicosanoid arachidonic acid to 20-Hydroxyeicosatetraenoic acid (20-HETE) is essential for activating the GPR75 receptor, leading to vasoconstriction and cell proliferation. Several mouse models of diet-induced MASLD have revealed the induction of selective CYP4A members and the suppression of CYP4F during steatosis and steatohepatitis, suggesting a critical metabolic role in the progression of fatty liver disease. Thus, to further investigate the functional roles of CYP4 genes, we analyzed the differential gene expression of 12 members of CYP4 gene family in datasets from the Gene Expression Omnibus (GEO) from patients with steatosis, steatohepatitis, fibrosis, cirrhosis, and hepatocellular carcinoma. We also observed the differential expression of various CYP4 genes in the progression of MASLD, indicating that different CYP4 members may have unique functional roles in the metabolism of specific FAs and eicosanoids at various stages of fatty liver disease. These results suggest that targeting selective members of the CYP4A family is a viable therapeutic approach for treating and managing MASLD.
Keywords: Arachidonic acid; Fatty liver disease; Fibrosis; cirrhosis. Steatosis; cytochrome P450; hepatocellular carcinoma (HCC); metabolic dysfunction-associated steatotic liver disease (MASLD).
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


















References
-
- Garcia V, Gilani A, Shkolnik B, Pandey V, Zhang FF, Dakarapu R, Gandham SK, Reddy NR, Graves JP, Gruzdev A, Zeldin DC, Capdevila JH, Falck JR, Schwartzman ML, 20-HETE Signals Through G-Protein-Coupled Receptor GPR75 (G(q)) to Affect Vascular Function and Trigger Hypertension, Circ. Res 120 (11) (2017) 1776–1788. - PMC - PubMed
-
- Buch S, Stickel F, Trepo E, Way M, Herrmann A, Nischalke HD, Brosch M, Rosendahl J, Berg T, Ridinger M, Rietschel M, McQuillin A, Frank J, Kiefer F, Schreiber S, Lieb W, Soyka M, Semmo N, Aigner E, Datz C, Schmelz R, Bruckner S, Zeissig S, Stephan AM, Wodarz N, Deviere J, Clumeck N, Sarrazin C, Lammert F, Gustot T, Deltenre P, Volzke H, Lerch MM, Mayerle J, Eyer F, Schafmayer C, Cichon S, Nothen MM, Nothnagel M, Ellinghaus D, Huse K, Franke A, Zopf S, Hellerbrand C, Moreno C, Franchimont D, Morgan MY, Hampe J, A genome-wide association study confirms PNPLA3 and identifies TM6SF2 and MBOAT7 as risk loci for alcohol-related cirrhosis, Nat. Genet 47 (12) (2015) 1443–1448. - PubMed
-
- Longo M, Meroni M, Paolini E, Erconi V, Carli F, Fortunato F, Ronchi D, Piciotti R, Sabatini S, Macchi C, Alisi A, Miele L, Soardo G, Comi GP, Valenti L, Ruscica M, Fracanzani AL, Gastaldelli A, Dongiovanni P, TM6SF2/PNPLA3/MBOAT7 Loss-of-Function Genetic Variants Impact on NAFLD Development and Progression Both in Patients and in In Vitro Models, Cell. Mol. Gastroenterol. Hepatol 13 (3) (2022) 759–788. - PMC - PubMed
-
- Mansoor S, Maheshwari A, Di Guglielmo M, Furuya K, Wang M, Crowgey E, Molle-Rios Z, He Z, The PNPLA3 rs738409 Variant but not MBOAT7 rs641738 is a Risk Factor for Nonalcoholic Fatty Liver Disease in Obese U.S, Children of Hispanic Ethnicity, Pediatr Gastroenterol Hepatol Nutr 24 (5) (2021) 455–469. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

