Toward understanding the cellular control of vertebrate mineralization: The potential role of mitochondria
- PMID: 38697384
- PMCID: PMC11251007
- DOI: 10.1016/j.bone.2024.117112
Toward understanding the cellular control of vertebrate mineralization: The potential role of mitochondria
Abstract
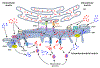
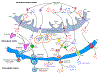
This review examines the possible role of mitochondria in maintaining calcium and phosphate ion homeostasis and participating in the mineralization of bone, cartilage and other vertebrate hard tissues. The paper builds on the known structural features of mitochondria and the documented observations in these tissues that the organelles contain calcium phosphate granules. Such deposits in mitochondria putatively form to buffer excessively high cytosolic calcium ion concentrations and prevent metabolic deficits and even cell death. While mitochondria protect cytosolic enzyme systems through this buffering capacity, the accumulation of calcium ions by mitochondria promotes the activity of enzymes of the tricarboxylic acid (TCA/Krebs) cycle, increases oxidative phosphorylation and ATP synthesis, and leads to changes in intramitochondrial pH. These pH alterations influence ion solubility and possibly the transitions and composition in the mineral phase structure of the granules. Based on these considerations, mitochondria are proposed to support the mineralization process by providing a mobile store of calcium and phosphate ions, in smaller cluster or larger granule form, while maintaining critical cellular activities. The rise in the mitochondrial calcium level also increases the generation of citrate and other TCA cycle intermediates that contribute to cell function and the development of extracellular mineral. This paper suggests that another key role of the mitochondrion, along with the effects just noted, is to supply phosphate ions, derived from the breakdown of ATP, to endolysosomes and autophagic vesicles originating in the endoplasmic reticulum and Golgi and at the plasma membrane. These many separate but interdependent mitochondrial functions emphasize the critical importance of this organelle in the cellular control of vertebrate mineralization.
Keywords: ATP; Calcium; Endoplasmic reticulum; Mineralization; Mitochondria; Mitochondrial granules; Phosphate.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest All authors have no conflicts to declare.
Figures

Similar articles
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Relationships between matrix mineralization, oxidative metabolism, and mitochondrial structure during ATDC5 murine chondroprogenitor cell line differentiation.J Cell Physiol. 2024 Aug;239(8):e31285. doi: 10.1002/jcp.31285. Epub 2024 Jun 11. J Cell Physiol. 2024. PMID: 38860464 Free PMC article.
-
A fuzzy based computational model to analyze the influence of mitochondria, buffer, and ER fluxes on cytosolic calcium distribution in neuron cells.Cogn Neurodyn. 2025 Dec;19(1):25. doi: 10.1007/s11571-024-10212-y. Epub 2025 Jan 13. Cogn Neurodyn. 2025. PMID: 39816214
-
Phosphate binders for preventing and treating chronic kidney disease-mineral and bone disorder (CKD-MBD).Cochrane Database Syst Rev. 2018 Aug 22;8(8):CD006023. doi: 10.1002/14651858.CD006023.pub3. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2025 Jun 27;6:CD006023. doi: 10.1002/14651858.CD006023.pub4. PMID: 30132304 Free PMC article. Updated.
Cited by
-
Identification of Biomarkers for Meat Quality in Sichuan Goats Through 4D Label-Free Quantitative Proteomics.Animals (Basel). 2025 Mar 20;15(6):887. doi: 10.3390/ani15060887. Animals (Basel). 2025. PMID: 40150416 Free PMC article.
-
Targeting mitochondria in bone and cartilage diseases: A narrative review.Redox Biol. 2025 Jun;83:103667. doi: 10.1016/j.redox.2025.103667. Epub 2025 May 7. Redox Biol. 2025. PMID: 40354767 Free PMC article. Review.
-
Axonal Energy Crisis and Calcium Phosphate Dysregulation as Pathogenesis of Optic Disc Drusen.Aging Dis. 2024 Sep 30;16(5):2739-2751. doi: 10.14336/AD.2024.0459. Aging Dis. 2024. PMID: 39500350 Free PMC article. Review.
-
Phosphate in Physiological and Pathological Mineralization: Important yet Often Unheeded.MedComm (2020). 2025 Jul 13;6(7):e70298. doi: 10.1002/mco2.70298. eCollection 2025 Jul. MedComm (2020). 2025. PMID: 40661139 Free PMC article. Review.
References
-
- van Leeuwenhoek A. Letter 39 in Collected Letters of 1676-1679, Vol. 8. Edited, illustrated and annotated by a committee of Dutch scientists (Swets & Zeitlinger, Amsterdam, 1941)
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

