Structural analysis of human IgE monoclonal antibody epitopes on dust mite allergen Der p 2
- PMID: 38697404
- PMCID: PMC11409219
- DOI: 10.1016/j.jaci.2024.04.017
Structural analysis of human IgE monoclonal antibody epitopes on dust mite allergen Der p 2
Abstract
Background: Human IgE (hIgE) mAbs against major mite allergen Der p 2 developed using human hybridoma technology were used for IgE epitope mapping and analysis of epitopes associated with the hIgE repertoire.
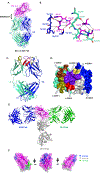
Objective: We sought to elucidate the new hIgE mAb 4C8 epitope on Der p 2 and compare it to the hIgE mAb 2F10 epitope in the context of the allergenic structure of Der p 2.
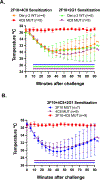
Methods: X-ray crystallography was used to determine the epitope of anti-Der p 2 hIgE mAb 4C8. Epitope mutants created by targeted mutagenesis were analyzed by immunoassays and in vivo using a human high-affinity IgE receptor (FcεRIα)-transgenic mouse model of passive systemic anaphylaxis.
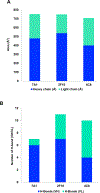
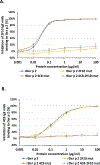
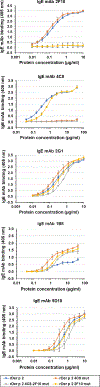
Results: The structure of recombinant Der p 2 with hIgE mAb 4C8 Fab was determined at 3.05 Å. The newly identified epitope region does not overlap with the hIgE mAb 2F10 epitope or the region recognized by 3 overlapping hIgE mAbs (1B8, 5D10, and 2G1). Compared with wild-type Der p 2, single or double 4C8 and 2F10 epitope mutants bound less IgE antibodies from allergic patients by as much as 93%. Human FcεRIα-transgenic mice sensitized by hIgE mAbs, which were susceptible to anaphylaxis when challenged with wild-type Der p 2, could no longer cross-link FcεRI to induce anaphylaxis when challenged with the epitope mutants.
Conclusions: These data establish the structural basis of allergenicity of 2 hIgE mAb nonoverlapping epitopes on Der p 2, which appear to make important contributions to the hIgE repertoire against Der p 2 and provide molecular targets for future design of allergy therapeutics.
Keywords: IgE; anaphylaxis; antibody; epitope; house dust mite.
Copyright © 2024 American Academy of Allergy, Asthma & Immunology. All rights reserved.
Conflict of interest statement
Disclosure statement Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) (award nos. R01AI077653-13 [to A.P., M.D.C., and M.C.] and R01AI155668 and R21AI123307 [to S.A.S.]). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The research was supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (Z01-ES102906 [to G.A.M.]). The structural results shown in this report are derived from data collected at Southeast Regional Collaborative Access Team (SER-CAT; 22ID) beamline at the Advanced Photon Source, Argonne National Laboratory. Supporting institutions may be found at www.ser-cat.org/members.html. Use of the Advanced Photon Source was supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences (contract nos. DE-AC02-06CH11357 and W-31-109-Eng-38). This work was partially supported by an ASPIRE III grant from the Office of the Vice President of Research at the University of South Carolina. This research is funded by the above mentioned NIH/NIAID award to InBio. Disclosure of potential conflict of interest: A. Pomés is an employee of InBio; and is the contact principal investigator of the NIH R01 award that provided funding for the study. M.D. Chapman is an employee of InBio; has a financial interest in InBio; and is a co-investigator on the NIH R01 award. A. Ball is an employee of InBio. The hIgE mAbs and some of the allergens described herein were produced by InBio. InBio has a license agreement with the VUMC for commercialization of hIgE mAbs for research and diagnostic purposes. The hIgE mAbs covered by this agreement are available from InBio (www.inbio.com). S.A. Smith is an inventor on US patent 10908168-B2 for generation of hIgE mAbs; has received patent royalties; and has related patents pending. The rest of the authors declare that they have no relevant conflicts of interest.
Figures

References
-
- Platts-Mills TA, Vervloet D, Thomas WR, Aalberse RC, Chapman MD. Indoor allergens and asthma: report of the third international workshop. J Allergy Clin Immunol. 1997;100(6 Pt 1):S2–24. - PubMed
-
- Batard T, Baron-Bodo V, Martelet A, Le Mignon M, Lemoine P, Jain K, et al. Patterns of IgE sensitization in house dust mite-allergic patients: implications for allergen immunotherapy. Allergy. 2016;71(2):220–9. - PubMed
-
- Posa D, Perna S, Resch Y, Lupinek C, Panetta V, Hofmaier S, et al. Evolution and predictive value of IgE responses toward a comprehensive panel of house dust mite allergens during the first 2 decades of life. J Allergy Clin Immunol. 2017;139(2):541–9 e8. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

