Population pharmacokinetic modelling and simulation of tranexamic acid in adult trauma patients
- PMID: 38697615
- PMCID: PMC11932107
- DOI: 10.1111/bcp.16075
Population pharmacokinetic modelling and simulation of tranexamic acid in adult trauma patients
Abstract
Aims: The aim of this study is to describe the disposition of tranexamic acid (TXA) in adult trauma patients and derive a dosing regimen that optimizes exposure based on a predefined exposure target.
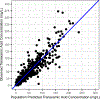
Methods: We performed a population pharmacokinetic (popPK) analysis of participants enrolled in the Tranexamic Acid Mechanisms and Pharmacokinetics in Traumatic Injury (TAMPITI) trial (≥18 years with traumatic injury, given ≥1 blood product and/or requiring immediate transfer to the operating room) who were randomized to a single dose of either 2 or 4 g of TXA ≤2 h from time of injury. PopPK analysis was conducted using nonlinear mixed-effects modelling (NONMEM). Simulations were then performed using the final model to generate estimated plasma TXA concentrations in 1000 simulated participants. Dosing schemes were evaluated to determine maintenance of TXA plasma concentrations >10 mg/L for ≥8 h after administration of the initial dose.
Results: TXA PK was best described by a two-compartment model with proportional residual error and allometric scaling on all parameters. Platelet count, skeletal muscle oxygen saturation measured by near-infrared spectroscopy and interleukin-8 concentration were significant covariates on TXA clearance. Based on simulations, a 2 g IV bolus dose, repeated 3 h later, best achieved the target exposure.
Conclusions: According to simulations from a popPK model of TXA, a 2 g IV bolus with a repeated dose 3 h later would be most likely to maintain concentrations >10 mg/L for 8 h in >95% of adult trauma patients and should be considered for patients with ongoing haemorrhage.
Keywords: clinical pharmacology; emergency medicine; pharmacokinetics.
© 2024 The Authors. British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
Conflict of Interest:
• The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


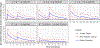
Similar articles
-
Population Pharmacokinetics of Tranexamic Acid in Chinese Population Undergoing Cardiac Surgery with Cardiopulmonary Bypass.Drug Des Devel Ther. 2025 May 26;19:4343-4353. doi: 10.2147/DDDT.S493485. eCollection 2025. Drug Des Devel Ther. 2025. PMID: 40453206 Free PMC article. Clinical Trial.
-
Antifibrinolytic drugs for acute traumatic injury.Cochrane Database Syst Rev. 2015 May 9;2015(5):CD004896. doi: 10.1002/14651858.CD004896.pub4. Cochrane Database Syst Rev. 2015. PMID: 25956410 Free PMC article.
-
Antifibrinolytic drugs for treating primary postpartum haemorrhage.Cochrane Database Syst Rev. 2018 Feb 20;2(2):CD012964. doi: 10.1002/14651858.CD012964. Cochrane Database Syst Rev. 2018. PMID: 29462500 Free PMC article.
-
Tranexamic acid for preventing postpartum haemorrhage after caesarean section.Cochrane Database Syst Rev. 2024 Nov 13;11(11):CD016278. doi: 10.1002/14651858.CD016278. Cochrane Database Syst Rev. 2024. PMID: 39535297 Free PMC article.
-
Pharmacological interventions for the prevention of bleeding in people undergoing elective hip or knee surgery: a systematic review and network meta-analysis.Cochrane Database Syst Rev. 2024 Jan 16;1(1):CD013295. doi: 10.1002/14651858.CD013295.pub2. Cochrane Database Syst Rev. 2024. PMID: 38226724 Free PMC article.
Cited by
-
A comprehensive review of massive transfusion and major hemorrhage protocols: origins, core principles and practical implementation.Braz J Anesthesiol. 2025 Mar-Apr;75(2):844583. doi: 10.1016/j.bjane.2024.844583. Epub 2024 Dec 25. Braz J Anesthesiol. 2025. PMID: 39730103 Free PMC article. Review.
-
Population pharmacokinetic model of tranexamic acid in patients who undergo cardiac surgery with cardiopulmonary bypass.Eur J Clin Pharmacol. 2025 Mar;81(3):441-449. doi: 10.1007/s00228-025-03802-0. Epub 2025 Jan 16. Eur J Clin Pharmacol. 2025. PMID: 39820511 Free PMC article.
References
-
- World Health Organisation, Health topics - injury, 2021. https://wjes.biomedcentral.com/articles/10.1186/s13017-020-00330-3 - DOI
-
- Committee on Military Trauma Care’s Learning Health System and Its Translation to the Civilian Sector, Board on Health Sciences Policy, Board on the Health of Select Populations, Health and Medicine Division, National Academies of Sciences, Engineering, and Medicine. A National Trauma Care System: Integrating Military and Civilian Trauma Systems to Achieve Zero Preventable Deaths After Injury. (Berwick D, Downey A, Cornett E, eds.). National Academies Press; 2016:23511. doi: 10.17226/23511 - DOI - PubMed
MeSH terms
Substances
Grants and funding
- K23-HD091365/Eunice Kennedy Shriver National Institute of Child Health and Human Development
- T32 GM008562/GM/NIGMS NIH HHS/United States
- W81XWH-14-1-0373/United States Department of Defense, United States Army Medical Research and Materiel Command
- K23 HD091365/HD/NICHD NIH HHS/United States
- T32GM008562/Eunice Kennedy Shriver National Institute of Child Health and Human Development
LinkOut - more resources
Full Text Sources
Medical

