VDR restores the expression of PINK1 and BNIP3 in TECs of streptozotocin-induced diabetic mice
- PMID: 38697845
- PMCID: PMC11066303
- DOI: 10.26508/lsa.202302474
VDR restores the expression of PINK1 and BNIP3 in TECs of streptozotocin-induced diabetic mice
Abstract
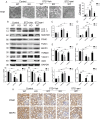
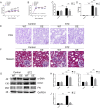
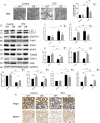
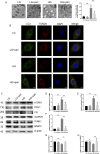
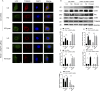
Defective mitophagy in renal tubular epithelial cells is one of the main drivers of renal fibrosis in diabetic kidney disease. Our gene sequencing data showed the expression of PINK1 and BNIP3, two key molecules of mitophagy, was decreased in renal tissues of VDR-knockout mice. Herein, streptozotocin (STZ) was used to induce renal interstitial fibrosis in mice. VDR deficiency exacerbated STZ-induced renal impairment and defective mitophagy. Paricalcitol (pari, a VDR agonist) and the tubular epithelial cell-specific overexpression of VDR restored the expression of PINK1 and BNIP3 in the renal cortex and attenuated STZ-induced kidney fibrosis and mitochondrial dysfunction. In HK-2 cells under high glucose conditions, an increased level of α-SMA, COL1, and FN and a decreased expression of PINK1 and BNIP3 with severe mitochondrial damage were observed, and these alterations could be largely reversed by pari treatment. ChIP-qPCR and luciferase reporter assays showed VDR could positively regulate the transcription of Pink1 and Bnip3 genes. These findings reveal that VDR could restore mitophagy defects and attenuate STZ-induced fibrosis in diabetic mice through regulation of PINK1 and BNIP3.
© 2024 Yang et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures






Similar articles
-
VDR regulates mitochondrial function as a protective mechanism against renal tubular cell injury in diabetic rats.Redox Biol. 2024 Apr;70:103062. doi: 10.1016/j.redox.2024.103062. Epub 2024 Jan 26. Redox Biol. 2024. PMID: 38320454 Free PMC article.
-
Vitamin D-VDR (vitamin D receptor) regulates defective autophagy in renal tubular epithelial cell in streptozotocin-induced diabetic mice via the AMPK pathway.Autophagy. 2022 Apr;18(4):877-890. doi: 10.1080/15548627.2021.1962681. Epub 2021 Aug 25. Autophagy. 2022. PMID: 34432556 Free PMC article.
-
Caffeic acid phenethyl ester protects renal tubular epithelial cells against ferroptosis in diabetic kidney disease via restoring PINK1-mediated mitophagy.Mol Med. 2025 Jul 24;31(1):264. doi: 10.1186/s10020-025-01318-y. Mol Med. 2025. PMID: 40702453 Free PMC article.
-
The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/PINK1.Redox Biol. 2017 Apr;11:297-311. doi: 10.1016/j.redox.2016.12.022. Epub 2016 Dec 21. Redox Biol. 2017. PMID: 28033563 Free PMC article.
-
The Mitochondrial Kinase PINK1 in Diabetic Kidney Disease.Int J Mol Sci. 2021 Feb 3;22(4):1525. doi: 10.3390/ijms22041525. Int J Mol Sci. 2021. PMID: 33546409 Free PMC article. Review.
Cited by
-
Altered mitochondrial function: a clue therapeutic strategies between metabolic dysfunction-associated steatotic liver disease and chronic kidney disease?Front Nutr. 2025 Jun 13;12:1613640. doi: 10.3389/fnut.2025.1613640. eCollection 2025. Front Nutr. 2025. PMID: 40584092 Free PMC article. Review.
-
Protective role of vitamin D receptor against mitochondrial calcium overload from PM2.5-Induced injury in renal tubular cells.Redox Biol. 2025 Mar;80:103518. doi: 10.1016/j.redox.2025.103518. Epub 2025 Jan 28. Redox Biol. 2025. PMID: 39891958 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
