MDM2 Inhibitors for Cancer Therapy: The Past, Present, and Future
- PMID: 38697854
- PMCID: PMC11068841
- DOI: 10.1124/pharmrev.123.001026
MDM2 Inhibitors for Cancer Therapy: The Past, Present, and Future
Abstract
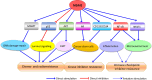
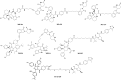
Since its discovery over 35 years ago, MDM2 has emerged as an attractive target for the development of cancer therapy. MDM2's activities extend from carcinogenesis to immunity to the response to various cancer therapies. Since the report of the first MDM2 inhibitor more than 30 years ago, various approaches to inhibit MDM2 have been attempted, with hundreds of small-molecule inhibitors evaluated in preclinical studies and numerous molecules tested in clinical trials. Although many MDM2 inhibitors and degraders have been evaluated in clinical trials, there is currently no Food and Drug Administration (FDA)-approved MDM2 inhibitor on the market. Nevertheless, there are several current clinical trials of promising agents that may overcome the past failures, including agents granted FDA orphan drug or fast-track status. We herein summarize the research efforts to discover and develop MDM2 inhibitors, focusing on those that induce MDM2 degradation and exert anticancer activity, regardless of the p53 status of the cancer. We also describe how preclinical and clinical investigations have moved toward combining MDM2 inhibitors with other agents, including immune checkpoint inhibitors. Finally, we discuss the current challenges and future directions to accelerate the clinical application of MDM2 inhibitors. In conclusion, targeting MDM2 remains a promising treatment approach, and targeting MDM2 for protein degradation represents a novel strategy to downregulate MDM2 without the side effects of the existing agents blocking p53-MDM2 binding. Additional preclinical and clinical investigations are needed to finally realize the full potential of MDM2 inhibition in treating cancer and other chronic diseases where MDM2 has been implicated. SIGNIFICANCE STATEMENT: Overexpression/amplification of the MDM2 oncogene has been detected in various human cancers and is associated with disease progression, treatment resistance, and poor patient outcomes. This article reviews the previous, current, and emerging MDM2-targeted therapies and summarizes the preclinical and clinical studies combining MDM2 inhibitors with chemotherapy and immunotherapy regimens. The findings of these contemporary studies may lead to safer and more effective treatments for patients with cancers overexpressing MDM2.
Copyright © 2024 by The Author(s).
Figures


References
-
- Abdul Razak AR, Bauer S, Suarez C, Lin CC, Quek R, Hütter-Krönke ML, Cubedo R, Ferretti S, Guerreiro N, Jullion A, et al. (2022) Co-Targeting of MDM2 and CDK4/6 with Siremadlin and Ribociclib for the Treatment of Patients with Well-Differentiated or Dedifferentiated Liposarcoma: Results from a Proof-of-Concept, Phase Ib Study. Clin Cancer Res 28:1087–1097. - PubMed
-
- Abdul Razak AR, Miller WH Jr, Uy GL, Blotner S, Young AM, Higgins B, Chen LC, Gore L (2020) A phase 1 study of the MDM2 antagonist RO6839921, a pegylated prodrug of idasanutlin, in patients with advanced solid tumors. Invest New Drugs 38:1156–1165. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

