Adrenoceptor Desensitization: Current Understanding of Mechanisms
- PMID: 38697858
- PMCID: PMC12164723
- DOI: 10.1124/pharmrev.123.000831
Adrenoceptor Desensitization: Current Understanding of Mechanisms
Abstract
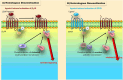
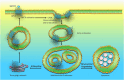
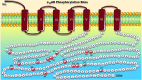
G-protein coupled receptors (GPCRs) transduce a wide range of extracellular signals. They are key players in the majority of biologic functions including vision, olfaction, chemotaxis, and immunity. However, as essential as most of them are to body function and homeostasis, overactivation of GPCRs has been implicated in many pathologic diseases such as cancer, asthma, and heart failure (HF). Therefore, an important feature of G protein signaling systems is the ability to control GPCR responsiveness, and one key process to control overstimulation involves initiating receptor desensitization. A number of steps are appreciated in the desensitization process, including cell surface receptor phosphorylation, internalization, and downregulation. Rapid or short-term desensitization occurs within minutes and involves receptor phosphorylation via the action of intracellular protein kinases, the binding of β-arrestins, and the consequent uncoupling of GPCRs from their cognate heterotrimeric G proteins. On the other hand, long-term desensitization occurs over hours to days and involves receptor downregulation or a decrease in cell surface receptor protein level. Of the proteins involved in this biologic phenomenon, β-arrestins play a particularly significant role in both short- and long-term desensitization mechanisms. In addition, β-arrestins are involved in the phenomenon of biased agonism, where the biased ligand preferentially activates one of several downstream signaling pathways, leading to altered cellular responses. In this context, this review discusses the different patterns of desensitization of the α 1-, α 2- and the β adrenoceptors and highlights the role of β-arrestins in regulating physiologic responsiveness through desensitization and biased agonism. SIGNIFICANCE STATEMENT: A sophisticated network of proteins orchestrates the molecular regulation of GPCR activity. Adrenoceptors are GPCRs that play vast roles in many physiological processes. Without tightly controlled desensitization of these receptors, homeostatic imbalance may ensue, thus precipitating various diseases. Here, we critically appraise the mechanisms implicated in adrenoceptor desensitization. A better understanding of these mechanisms helps identify new druggable targets within the GPCR desensitization machinery and opens exciting therapeutic fronts in the treatment of several pathologies.
Copyright © 2024 by The American Society for Pharmacology and Experimental Therapeutics.
Figures









References
-
- Aggarwal A, Esler MD, Socratous F, Kaye DM. Evidence for functional presynaptic alpha-2 adrenoceptors and their down-regulation in human heart failure. J Am Coll Cardiol. 2001;37:1246–1251. - PubMed
-
- Ahn S, Shenoy SK, Wei H, Lefkowitz RJ. Differential kinetic and spatial patterns of β-arrestin and G protein-mediated ERK activation by the angiotensin II receptor. J Biol Chem. 2004;279:35518–35525. - PubMed
-
- Akinaga J, Lima V, Kiguti LR, Hebeler-Barbosa F, Alcántara-Hernández R, García-Sáinz JA, Pupo AS. Differential phosphorylation, desensitization, and internalization of α1A-adrenoceptors activated by norepinephrine and oxymetazoline. Mol Pharmacol. 2013;83:870–881. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
