The complete assembly of human LAT1-4F2hc complex provides insights into its regulation, function and localisation
- PMID: 38697966
- PMCID: PMC11065870
- DOI: 10.1038/s41467-024-47948-4
The complete assembly of human LAT1-4F2hc complex provides insights into its regulation, function and localisation
Abstract
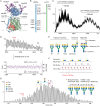
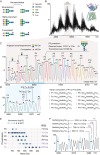
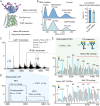
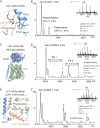
The LAT1-4F2hc complex (SLC7A5-SLC3A2) facilitates uptake of essential amino acids, hormones and drugs. Its dysfunction is associated with many cancers and immune/neurological disorders. Here, we apply native mass spectrometry (MS)-based approaches to provide evidence of super-dimer formation (LAT1-4F2hc)2. When combined with lipidomics, and site-directed mutagenesis, we discover four endogenous phosphatidylethanolamine (PE) molecules at the interface and C-terminus of both LAT1 subunits. We find that interfacial PE binding is regulated by 4F2hc-R183 and is critical for regulation of palmitoylation on neighbouring LAT1-C187. Combining native MS with mass photometry (MP), we reveal that super-dimerization is sensitive to pH, and modulated by complex N-glycans on the 4F2hc subunit. We further validate the dynamic assemblies of LAT1-4F2hc on plasma membrane and in the lysosome. Together our results link PTM and lipid binding with regulation and localisation of the LAT1-4F2hc super-dimer.
© 2024. The Author(s).
Conflict of interest statement
C.V.R. is a cofounder of and consultant at OMass Therapeutics. The remaining authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

