A novel inhibitor of the mitochondrial respiratory complex I with uncoupling properties exerts potent antitumor activity
- PMID: 38697987
- PMCID: PMC11065874
- DOI: 10.1038/s41419-024-06668-9
A novel inhibitor of the mitochondrial respiratory complex I with uncoupling properties exerts potent antitumor activity
Abstract
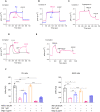
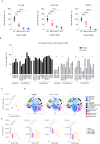
Cancer cells are highly dependent on bioenergetic processes to support their growth and survival. Disruption of metabolic pathways, particularly by targeting the mitochondrial electron transport chain complexes (ETC-I to V) has become an attractive therapeutic strategy. As a result, the search for clinically effective new respiratory chain inhibitors with minimized adverse effects is a major goal. Here, we characterize a new OXPHOS inhibitor compound called MS-L6, which behaves as an inhibitor of ETC-I, combining inhibition of NADH oxidation and uncoupling effect. MS-L6 is effective on both intact and sub-mitochondrial particles, indicating that its efficacy does not depend on its accumulation within the mitochondria. MS-L6 reduces ATP synthesis and induces a metabolic shift with increased glucose consumption and lactate production in cancer cell lines. MS-L6 either dose-dependently inhibits cell proliferation or induces cell death in a variety of cancer cell lines, including B-cell and T-cell lymphomas as well as pediatric sarcoma. Ectopic expression of Saccharomyces cerevisiae NADH dehydrogenase (NDI-1) partially restores the viability of B-lymphoma cells treated with MS-L6, demonstrating that the inhibition of NADH oxidation is functionally linked to its cytotoxic effect. Furthermore, MS-L6 administration induces robust inhibition of lymphoma tumor growth in two murine xenograft models without toxicity. Thus, our data present MS-L6 as an inhibitor of OXPHOS, with a dual mechanism of action on the respiratory chain and with potent antitumor properties in preclinical models, positioning it as the pioneering member of a promising drug class to be evaluated for cancer therapy. MS-L6 exerts dual mitochondrial effects: ETC-I inhibition and uncoupling of OXPHOS. In cancer cells, MS-L6 inhibited ETC-I at least 5 times more than in isolated rat hepatocytes. These mitochondrial effects lead to energy collapse in cancer cells, resulting in proliferation arrest and cell death. In contrast, hepatocytes which completely and rapidly inactivated this molecule, restored their energy status and survived exposure to MS-L6 without apparent toxicity.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

