Cognitive impairment and hippocampal neuronal damage in β-thalassaemia mice
- PMID: 38698053
- PMCID: PMC11066061
- DOI: 10.1038/s41598-024-60459-y
Cognitive impairment and hippocampal neuronal damage in β-thalassaemia mice
Abstract
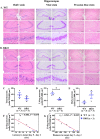
β-Thalassaemia is one of the most common genetic diseases worldwide. During the past few decades, life expectancy of patients has increased significantly owing to advance in medical treatments. Cognitive impairment, once has been neglected, has gradually become more documented. Cognitive impairment in β-thalassaemia patients is associated with natural history of the disease and socioeconomic factors. Herein, to determined effect of β-thalassaemia intrinsic factors, 22-month-old β-thalassaemia mouse was used as a model to assess cognitive impairment and to investigate any aberrant brain pathology in β-thalassaemia. Open field test showed that β-thalassaemia mice had decreased motor function. However, no difference of neuronal degeneration in primary motor cortex, layer 2/3 area was found. Interestingly, impaired learning and memory function accessed by a Morris water maze test was observed and correlated with a reduced number of living pyramidal neurons in hippocampus at the CA3 region in β-thalassaemia mice. Cognitive impairment in β-thalassaemia mice was significantly correlated with several intrinsic β-thalassaemic factors including iron overload, anaemia, damaged red blood cells (RBCs), phosphatidylserine (PS)-exposed RBC large extracellular vesicles (EVs) and PS-exposed medium EVs. This highlights the importance of blood transfusion and iron chelation in β-thalassaemia patients. In addition, to improve patients' quality of life, assessment of cognitive functions should become part of routine follow-up.
Keywords: Aged mouse model; Behavior test; Brain pathology; Cognitive impairment; Hippocampus; β-thalassaemia.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Grants and funding
- Grant no. PHD/0054/2561, Code no. 4.U.MU/61/C.1.O.XX/the Royal Golden Jubilee PhD Research Scholarship, the National Research Council of Thailand (NRCT) and the Thailand Research Fund (TRF)
- MRC-MGR 01/2565/Mahidol University
- Basic Research Fund: fiscal year 2022/Mahidol University
- BRF1-077/2565/Mahidol University
- N42A650349/National Research Council of Thailand (NRCT) and Mahidol University
LinkOut - more resources
Full Text Sources
Miscellaneous

