Transient pacing in pigs with complete heart block via myocardial injection of mRNA coding for the T-box transcription factor 18
- PMID: 38698155
- PMCID: PMC11410671
- DOI: 10.1038/s41551-024-01211-9
Transient pacing in pigs with complete heart block via myocardial injection of mRNA coding for the T-box transcription factor 18
Abstract
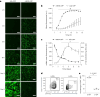
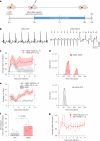
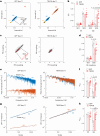
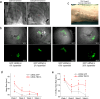
The adenovirus-mediated somatic transfer of the embryonic T-box transcription factor 18 (TBX18) gene can convert chamber cardiomyocytes into induced pacemaker cells. However, the translation of therapeutic TBX18-induced cardiac pacing faces safety challenges. Here we show that the myocardial expression of synthetic TBX18 mRNA in animals generates de novo pacing and limits innate and inflammatory immune responses. In rats, intramyocardially injected mRNA remained localized, whereas direct myocardial injection of an adenovirus carrying a reporter gene resulted in diffuse expression and in substantial spillover to the liver, spleen and lungs. Transient expression of TBX18 mRNA in rats led to de novo automaticity and pacemaker properties and, compared with the injection of adenovirus, to substantial reductions in the expression of inflammatory genes and in activated macrophage populations. In rodent and clinically relevant porcine models of complete heart block, intramyocardially injected TBX18 mRNA provided rate-adaptive cardiac pacing for one month that strongly correlated with the animal's sinus rhythm and physical activity. TBX18 mRNA may aid the development of biological pacemakers.
© 2024. The Author(s).
Conflict of interest statement
The authors have filed a patent (WO2019169228) related to this work.
Figures













References
-
- Villain, E. Indications for pacing in patients with congenital heart disease. Pacing Clin. Electrophysiol.31, S17–S20 (2008). - PubMed
-
- Connolly, S. J. et al. Effects of physiologic pacing versus ventricular pacing on the risk of stroke and death due to cardiovascular causes. Canadian Trial of Physiologic Pacing Investigators. N. Engl. J. Med.342, 1385–1391 (2000). - PubMed
-
- Amin, M. S., Matchar, D. B., Wood, M. A. & Ellenbogen, K. A. Management of recalled pacemakers and implantable cardioverter-defibrillators: a decision analysis model. JAMA296, 412–420 (2006). - PubMed
-
- Fortescue, E. B. et al. Patient, procedural, and hardware factors associated with pacemaker lead failures in pediatrics and congenital heart disease. Heart Rhythm1, 150–159 (2004). - PubMed
-
- Hauser, R. G. et al. Clinical experience with pacemaker pulse generators and transvenous leads: an 8-year prospective multicenter study. Heart Rhythm4, 154–160 (2007). - PubMed
MeSH terms
Substances
Grants and funding
- AHA19POST34450268/American Heart Association (American Heart Association, Inc.)
- R01HL111646/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- F31 HL149272/HL/NHLBI NIH HHS/United States
- R01 HL157363/HL/NHLBI NIH HHS/United States
- R01 AR080169/AR/NIAMS NIH HHS/United States
- R01HL143065/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R01 HL143065/HL/NHLBI NIH HHS/United States
- R01 HL161366/HL/NHLBI NIH HHS/United States
- R01 HL147270/HL/NHLBI NIH HHS/United States
- T32 GM145735/GM/NIGMS NIH HHS/United States
- F31HL149272/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
LinkOut - more resources
Full Text Sources

